Research article / Open Access
DOI: 10.31488/bjcr.189
A Phase 1b Study Of CBP501, a Novel Immunogenic Cell Death Inducer, Combined with Cisplatin and Nivolumab in Patients with Advanced Solid Tumors
Marc Matrana1, Frank Tsai2, Suma Satti1, Erkut H. Borazanci2, Justin C. Moser2, Khanh Do3, Lingling Du1, Vivek Khemka2, Valentin Kolmakov4, Takumi Kawabe5, Donald W. Kufe3, Daniel D. Von Hoff2, Geoffrey I. Shapiro*3
1. Ochsner Clinic Foundation, New Orleans, LA, USA
2. HonorHealth Research Institute, Scottsdale, AZ, USA
3. Dana-Farber Cancer Institute, Boston, MA, USA
4. Parexel International, Durham, NC, USA
5. CanBas Co., Ltd, Numazu City, Japan
*Corresponding author: Geoffrey I. Shapiro, Dana-Farber Cancer Institute, Boston, MA, USA
Abstract
Background: In preclinical models, CBP501 induces immunogenic tumor cell death and CD8+ T-cell infiltration into tumors in combination with cisplatin and increases the efficacy of immune checkpoint inhibitors. This study evaluated the safety and efficacy of the combination of CBP501 + cisplatin + nivolumab in patients with advanced solid tumors. Methods: Patients (n=47) received concurrent intravenous (IV) infusions of CBP501 (16 or 25 mg/m2) and cisplatin (60 or 75 mg/m2), followed by an IV infusion of nivolumab (240 mg) once every 21 days in an open-label, multicenter 3 + 3 dose escalation trial with two dose confirmation cohorts, including advanced pancreatic cancer and microsatellite stable (MSS) colorectal cancer. Results: The recommended dose (RD) for expansion was 25 mg/m2 CBP501/ 60 mg/m2 cisplatin/ 240 mg nivolumab. The most common treatment-related adverse events (TRAEs), occurring in ≥20% of patients, at the RD (n=35) were infusion-related reactions (80%), fatigue (49%), anemia (46%), leukopenia (34%), neutropenia (26%), thrombocytopenia (23%), and increased ALT (20%). Of 24 patients with advanced pancreatic cancer, 6 patients achieved SD as best response; the median PFS and OS among patients with SD was 5.0 and 6.3 months, respectively, and greatest benefit was seen in patients with WBC ≤ 10,000/mm3. Conclusions: CBP501 combined with cisplatin and nivolumab demonstrated a manageable safety profile, as well as preliminary evidence of clinical benefit as ≥ 3rd line therapy for advanced pancreatic cancer. These findings support initiation of a randomized phase 2 study in this population.
Background
CBP501 is a unique cell-permeable peptide that binds to calmodulin and has been shown to enhance accretion of platinum-based chemotherapy by tumor cells [1]. In addition, CBP501 has been shown to induce tumor immunogenic cell death [2], suppress the function of tumor-associated macrophages [3], reduce cancer stem cell populations [3], and reduce migration and invasion by inhibiting the epithelial-to-mesenchymal transition of tumor cells [4]. In in vivo cancer models, CBP501 induced immunogenic tumor cell death and CD8+ T-cell infiltration into tumors in combination with cisplatin and increased the efficacy of immune checkpoint blockade [2].
Phase 1 studies in advanced solid tumors showed that CBP501 is well tolerated as monotherapy and with cisplatin [5]. Signs of antitumor activity were observed with CBP501/cisplatin plus/minus pemetrexed, in patients with platinum-resistant ovarian cancer (NCT00551512) [5], treatment-naive malignant pleural mesothelioma (NCT00700336) [6] and non-squamous non-small cell lung cancer (NCT00942825).
Nivolumab, a monoclonal antibody that blocks the human programmed death receptor 1 (PD-1), has been approved by the FDA for treatment of patients with a wide range of cancers. Additionally, phase 3 studies showed improved efficacy for platinum-based doublet chemotherapy combined with nivolumab or pembrolizumab in patients with advanced non-small cell lung cancer [7,8]. However, there are many patients who do not benefit from anti-PD-1-based treatment, suggesting a need for additional strategies to sensitize tumors to immune checkpoint blockade.
The induction of immunogenic cell death by the combination of CBP501 and cisplatin [2] and the suppression of M2 macrophages by CBP501 [3] are biological effects expected to render tumors sensitive to anti-PD-1-based treatment. Consistent with these effects, treatment with CBP501/cisplatin/nivolumab was significantly more effective than CBP501/cisplatin, cisplatin/nivolumab or any single agent in the CT26 syngeneic colon tumor model [2].
Based on this rationale, we conducted a phase 1, open-label, multicenter 3 + 3 dose escalation trial with dose confirmation cohorts to explore the safety of CBP501/cisplatin/nivolumab combination therapy in patients with advanced solid tumors and to assess preliminary evidence of efficacy in advanced pancreatic and MSS colorectal cancer. Pancreatic cancer was chosen based on unmet medical need in the second- and third-line setting. Because of the lack of expected activity for cisplatin or nivolumab monotherapies in both of these diseases [9,10], preliminary signs of clinical beneft could suggest a contribution of the modulatory effects of CBP501.
Methods
Study design
This was a phase 1, open-label, multicenter 3 + 3 dose escalation trial with two dose confirmation cohorts (NCT03113188), designed and sponsored by CanBas Co., Ltd. The study was conducted in accordance with the Declaration of Helsinki and was approved by local or central Institutional Review Boards at each investigational site. All patients provided written informed consent to participate in the study.
Patient selection
All participants were ≥18 years and had (1) previously treated, pathologically confirmed, locally advanced or metastatic solid tumors with measurable disease by RECIST v1.1 for which cisplatin was a reasonable treatment option; (2) no more than 2 prior lines incorporating immune checkpoint blockade; (3) ECOG performance status 0-1; and (4) sufficient bone marrow, renal and liver function. Patients with stable brain metastases were eligible if active treatment was not required at time of screening.
Study objectives
The primary objective of the study was to determine an RD for expansion. Secondary objectives were to characterize safety and tolerability of the combination therapy and to evaluate evidence of antitumor tumor activity.
Treatment plan
Appropriate prophylactic medications for cisplatin-induced kidney injury and emesis and CBP501-related infusion reactions were given prior to each administration of study drugs. For dose escalation cohorts, patients received concurrent IV infusions of CBP501 (16 or 25 mg/m2) and cisplatin (60 or 75 mg/m2) with the following hydration protocol or similar hydration protocols routinely administered at investigational sites: (1) 1.0 L of 0.9% Sodium Chloride Infusion with 2 g magnesium sulfate at 500 mL/hour; (2) 12.5 g of Mannitol by IV bolus injection after administration of 1 hour of hydration; (3) initiation of CBP501 and cisplatin infusions after completion of mannitol while continuing hydration; (4) urinary output of 250 mL/hour was maintained over the duration of the hydration, with additional mannitol (12.5 to 50.0 g via IV bolus injection) administered as needed. The CBP501/cisplatin infusions were followed by an IV infusion of nivolumab (240 mg). Treatment was administered once every 21 days, continuing until disease progression, intolerable toxicity, withdrawal of consent, or investigator decision. For dose expansion cohorts treated at the RD, the protocol was modified to restrict to 10 or fewer prior lines of therapy and to specify administration of a maximum of 4 cycles of CBP501 and cisplatin and a maximum of 10 cycles of nivolumab. This modification was inspired in part by the success of CheckMate 9LA [7], where nivolumab plus ipilimumab was combined with two cycles of chemotherapy followed by up to 10 cycles of nivolumab in the first-line treatment for patients with advanced non-small cell lung cancer. The addition of immunotherapy provided a significant improvement in overall survival versus chemotherapy alone, and demonstrated a favorable risk–benefit profile. Additionally, if CBP501 and/or cisplatin infusions had to be discontinued due to treatment-related toxicity, patients could continue to receive the other test drugs for a maximum of 4 cycles of CBP501 or cisplatin and a maximum of 10 cycles of nivolumab in total until discontinuation criteria were met.
Safety assessments
Regular safety assessments were performed, including physical examination, ECOG performance status, vital signs, laboratory parameters, and cardiac assessments. Adverse events (AEs) were assessed at each visit and assigned a grade, defined by the National Cancer Institute Common Terminology Criteria for Adverse Events v.4.03, and relationship to study treatment (i.e., related or unrelated for each drug in the combination). A clinical safety committee adjudicated dose-limiting toxicities (DLTs), dose escalations and de-escalations, RD, and maximum tolerated dose (MTD). All patients who received at least one study treatment were included in the safety analyses (“Safety Population”).
Biomarker evaluation
Pre-treatment and on-treatment cycle 2 biopsies were required of all patients enrolled to the expansion cohorts. An archival sample could substitute for the pretreatment biopsy if procured within one year prior to enrollment with no intervening treatment. PD-L1 immunohistochemistry was performed on fresh or archival biopsies with antibody clone 28-8 on 28-8 IHC pharmDx or clone SP142 on the Dako autostainer platform, and CD8+ T-cell immunohistochemistry was performed with antibody clone SP57 on the Ventana Benchmark XT autostainer platform. PD-L1 status was defined in tumor cells (TCs).
Efficacy assessments
Tumor assessment using RECIST v1.1 criteria was performed at screening, after every two cycles of treatment, and every three months from end of treatment to disease progression; all patients with at least one post-baseline tumor assessment were included in assessments of objective responses and progression-free survival (PFS) (“Efficacy Population”). Overall survival was analyzed for the entire safety population, the entire efficacy population, as well as for the safety and efficacy populations of patients with pancreatic cancer or MSS colon cancer, and patients with SD in the pancreatic cancer cohort.
Overall survival was also assessed in the subsets of these populations with WBC ≤ 10,000/mm3 at screening. These data were based on analyses performed for patients enrolled in previous clinical studies with CBP501, including CBP06-01 (Ovarian cancer cohort of Phase I; NCT00551512), CBP08-01 (Malignant pleural mesothelioma Phase I/II; NCT00700336), and CBP08-02 (Non-squamous non-small cell lung cancer Phase II; NCT00942825), where PFS and OS were determined for patient subpopulations with WBC ≤ 10,000 and >10,000/mm3 at screening.
Statistical Analysis
Quantitative variables were analysed using descriptive statistics. Continuous variables were analyzed as N, mean and/or median, standard deviation, range. Categorical variables were analysed using frequencies and percentage. A conventional algorithm (3+3 subjects per dose level) was used to identify the MTD, escalating if 0 of 3 or 1 of 6 DLTs were encountered, and de-escalating if 2 DLTs were encountered. PFS and OS analyses in relation to WBC count were performed with Statistical Analysis Software (SAS) and GraphPad Prism 9 and were ad-hoc for studies CBP06-01 and CBP08-01and preplanned for studies CBP08-02 and this study (CBP17-01). OS and PFS for patients with SD in pancreatic cancer were calculated using survival package of R.
Results
Patients
Between October 2017 and November 2020, 47 patients were enrolled and treated with at least one infusion of study drugs (Figure 1). Dose escalation cohorts of 3-6 patients evaluated dose levels of CBP501 (mg/m2)/ cisplatin (mg/m2)/ nivolumab (mg) of 16/60/240, 16/75/240, 25/60/240 and 25/75/240. Twenty-eight additional patients were treated at the RD (25/60/240) in dose confirmation cohorts of exocrine pancreatic cancer (N=19) and MSS colorectal cancer (N=9) to collect additional safety and efficacy data.
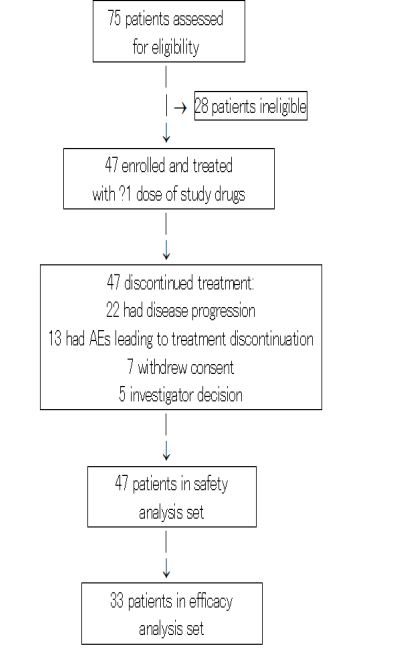
Figure 1: Trial profile
Patient demographics and baseline characteristics are summarized in table 1. Median age was 62.5 years; 38% of patients were ≥65 years old. At study entry, most patients (97.9%) had stage IV disease and multiple sites of disease (78.7%), including liver (57.4%), lung (27.7%) and lymph node metastases (19.1%). The median number of prior systemic therapies was three (range: 1-11); 27.7% of patients’ tumors were refractory to all prior treatments and only 19.1% had an objective response (complete response [CR] or partial response [PR]) to at least one prior line of systemic therapy.
Table 1.Patient demographics and baseline characteristics by treatment group
| Dose Level: CBP501 (mg/m2)/cisplatin (mg/m2)/nivolumab (mg) | Total N=47 | ||||
|---|---|---|---|---|---|
| 16/ 60/ 240 N=3 | 16/ 75/ 240 N=6 | 25/ 60/ 240 N=35 | 25/ 75/ 240 N=3 | ||
| Median age, years (range) | 56 (46-59) | 66.5 (56-76) | 63 (36-77) | 67 (61-69) | 62.5 (46-77) |
| Sex (male), n (%) | 2 (67) | 1 (17) | 18 (51) | 2 (67) | 23 (49) |
| Race, n (%) | |||||
| Caucasian | 1 (33) | 6 (100) | 28 (80) | 2 (67) | 37 (79) |
| Black | 2 (67) | 0 | 4 (11) | 1 (33) | 7 (15) |
| Other | 0 | 0 | 3 (9) | 0 | 3 (6) |
| ECOG PS, n (%) | |||||
| 0 | 1 | 4 | 17 (49) | 1 | 23 (49) |
| 1 | 2 | 2 | 18 (51) | 2 | 24 (51) |
| Primary cancer site, n (%) | |||||
| Colorectal | 0 | 3 | 10 (29) | 1 | 14 (30) |
| Pancreas | 1 | 1 | 20 (57) | 2 | 24 (51) |
| OtherA | 2 | 2 | 5 (14) | 0 | 9 (19) |
| Sites of metastases, n (%) | |||||
| #Patients with metastases | 3 | 6 | 35 (100) | 3 | 47 (100) |
| Liver | 1 | 2 | 23 (66) | 1 | 27 (57) |
| Lung | 2 | 1 | 9 (26) | 1 | 13 (28) |
| Lymph nodes | 0 | 3 | 5 (14) | 1 | 9 (19) |
| Other sitesB | 1 | 0 | 8 (23) | 0 | 9 (19) |
| Prior treatment regimens, n (%) | |||||
| Surgery | 2 | 3 | 18 (51) | 2 | 25 (53) |
| Chemotherapy | 3 | 6 | 34 (97) | 3 | 46 (98) |
| Immunotherapy | 0 | 2 | 3 (9) | 0 | 5 (11) |
| Prior chemo/immunotherapy | |||||
| # lines, median (range) | 4 (2-9) | 5 (1-11) | 3 (1-9) | 3 (2-6) | 3 (1-11) |
| Best response, n (%) | |||||
| CR (complete response) | 1 | 1 | 0 | 0 | 2 (4) |
| PR (partial response) | 0 | 3 | 4 (11) | 0 | 7 (15) |
| SD (stable disease) | 2 | 1 | 14 (40) | 2 | 19 (40) |
| PD (progressive disease | 0 | 1 | 11 (31) | 1 | 13 (28) |
| unknown | 0 | 0 | 6 (17) | 0 | 6 (13) |
A: breast (2), gall bladder (1), liver (1), ovary (3), thymus (1)
B: adrenal, bladder wall, kidney, pelvis, ovary, peritoneum, mesentery, spleen
Safety and tolerability
None of the 19 patients enrolled in dose escalation cohorts experienced a DLT during their first cycle of treatment. Two out of 6 patients discontinued treatment due to acute kidney injury at CBP501 16 mg/m2/cisplatin 75 mg/m2/nivolumab 240 mg. The RD was determined to be 25 mg/m2 CBP501/60 mg/m2 cisplatin/240 mg nivolumab. Among 35 patients treated at the RD, 4 (11%) had CBP501 dose reduced and 7 (20%) had cisplatin dose reduced over the course of treatment. (Supplementary Table 1).
All patients experienced at least one AE regardless of study drug relationship and 81% experienced a Grade 3-4 AE (Supplementary Table 2); no Grade 5 AEs were noted. TRAEs were noted for 100% of patients and 45% experienced a Grade 3-4 TRAE (all Grade 3, except for one Grade 4 event of acute kidney injury) (Table 2). At the RD (n=35), 100% of patients had a TRAE and 43% had a Grade 3 TRAE; no Grade 4-5 TRAEs were noted. The most common (≥20%) TRAEs at the RD were infusion-related reaction (80%), fatigue (49%), anemia (46%), leukopenia (34%), neutropenia (26%), thrombocytopenia (23%), and increased ALT (20%). The only Grade 3 TRAEs at the RD occurring in 2 or more (≥6%) patients were anemia (20%), leukopenia (9%), neutropenia (9%), fatigue (6%), increased ALT (6%), and acute kidney injury (6%).
Table 2.Treatment related adverse events (TRAEs) occurring in ≥10% of patients
| MedDRA SOC Preferred Term | Dose Level: CBP501 (mg/m2)/ cisplatin (mg/m2)/ nivolumab (mg) | Total N=47 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 16/ 60/ 240 N=3 | 25/ 60/ 240 N=35 | 25/ 75/ 240 N=3 | |||||||||
| Total | Gr 3 | Total | Gr 3 | Total | Gr 3 | Total | Gr 3 | Total | Gr 3 | ||
| n (%) | |||||||||||
| All TRAEs | 3 | 2 | 6 | 3 | 35 (100) | 15 (43) | 3 | 1 | 47 (100) | 21 (45) | |
| Blood and lymphatic system disorders | |||||||||||
| Anemia | 1 | 1 | 5 | 2 | 16 (46) | 7 (20) | 2 | 0 | 24 (51) | 10 (21) | |
| Leukopenia A | 1 | 0 | 1 | 0 | 12 (34) | 3 (9) | 1 | 0 | 15 (32) | 3 (6) | |
| Neutropenia B | 0 | 0 | 2 | 0 | 9 (26) | 3 (9) | 1 | 0 | 12 (26) | 3 (6) | |
| Thrombocytopenia C | 0 | 0 | 3 | 0 | 8 (23) | 1(3) | 1 | 0 | 12 (36) | 1 (2) | |
| Gastrointestinal disorders | |||||||||||
| Nausea | 0 | 0 | 3 | 0 | 4 (11) | 0 | 3 | 0 | 10 (21) | 0 | |
| Vomiting | 0 | 0 | 1 | 0 | 2 (6) | 0 | 2 | 0 | 5 (11) | 0 | |
| General disorders | |||||||||||
| Fatigue | 1 | 1 | 2 | 0 | 17 (49) | 2 (6) | 2 | 0 | 22 (47) | 3 (6 | |
| Injury, poisoning & procedural complications | |||||||||||
| Infusion related reaction D | 3 | 1 | 5 | 0 | 28 (80) | 1 (3) | 3 | 0 | 39 (83) | 2 (4) | |
| Investigations | |||||||||||
| ALT increased | 0 | 0 | 1 | 0 | 7 (20) | 2 (6) | 0 | 0 | 8 (17) | 2 (4) | |
| AST increased | 0 | 0 | 0 | 0 | 4 (11) | 1 (3) | 1 | 1 | 5 (11) | 2 (4) | |
| BUN increased | 1 | 0 | 3 | 0 | 2 (6) | 0 | 1 | 0 | 7 (15) | 0 | |
| Metabolism and nutrition disorders | |||||||||||
| Decreased appetite | 1 | 0 | 1 | 0 | 3 (9) | 0 | 1 | 0 | 6 (13) | 0 | |
| Hyponatremia | 1 | 0 | 0 | 0 | 3 (9) | 1 (3) | 1 | 0 | 5 (11) | 1 (2) | |
| Renal and urinary disorders | |||||||||||
| Acute kidney injury E1 | 1 | 0 | 2 | 1 | 3 (9) | 2 (6) | 1 | 0 | 7 (15) | 3 (6) | |
*Grade 4 event
A: Includes cases based on WBC decreased
B: Includes cases based on granulocyte or neutrophil count decreased
C: Includes cases based on platelet count decreased
D: Includes cases for anaphylactic reaction, cytokine release syndrome, urticaria, erythema, pruritis, flushing, rash
E: Includes cases based on blood creatinine increased
ALT: alanine aminotransferase; AST: aspartate aminotransferase; BUN: blood urea nitrogen
Overall, 23% (11/47) of patients experienced AEs leading to treatment discontinuation; 5 of these 11 events were considered unrelated to study treatment. The only AE leading to treatment discontinuation in more than one patient was acute kidney injury or the equivalent laboratory abnormality of decreased glomerular filtration rate in 5 patients. Only 3 AEs leading to treatment discontinuation were considered related to CBP501 (alone or combined with cisplatin), including one case each of infusion-related reaction, acute kidney injury, and decreased glomerular filtration rate.
Overall, 64% (30/47) of patients experienced one or more AEs leading to dose adjustment. AEs that led to dose adjustment in more than one patient were infusion-related reaction (13 patients), acute kidney injury with creatinine elevation and decreased glomerular filtration rate (7), fatigue (6), neutropenia, nausea (5) and hyponatremia (4).
Efficacy
Seventy-percent (33/47) of patients, including 58% (14/24) of pancreatic cancer patients and 86% (12/14) of colorectal cancer patients, were evaluable for assessment of objective response and PFS (Table 3). Two unconfirmed PRs were observed in patients with colorectal cancer and cholangiocarcinoma, respectively, both treated in the dose-escalation portion of the study with 16 mg/m2 CBP501/75 mg/m2 cisplatin/240 mg nivolumab. One pancreatic cancer patient experienced sustained regression of multiple hepatic lesions, who was treated in the dose-escalation cohort with 16 mg/m2 CBP501/60 mg/m2 cisplatin/240 mg nivolumab (Supplementary Figure 1).
Table 3. Efficacy Assessments
| Efficacy Population | Safety Population | ||||||
|---|---|---|---|---|---|---|---|
| Total N=33 | Pancreatic Cancer N=14 |
Pancreatic Cancer with SD N=6 | Colorectal
Cancer N=12 |
Total N=47 | Pancreatic Cancer N=24 | Colorectal Cancer N=14 | |
| Objective responses, n (%) | |||||||
| CR (complete response) | 0 | 0 | 0 | ||||
| PR (partial response) | 2 (6) | 0 | 1 (8.3) | ||||
| SD (stable disease) >12 weeks | 9 (27) | 6 (43) | 6 | 1 (8.3) | |||
| PD (progressive disease) | 22 (67) | 8 (57) | 10 (83) | ||||
| Disease control rate (PR+SD >12 weeks) |
11 (33) | 6 (43) | 2 (17) | ||||
| Progression-free survival (PFS), median (95% CI), months |
2.5 (1.4, 3.0) |
2.4 (1.3, 4.6) |
5 (4.2, NA) |
1.4 (1.3, 2.7) |
|||
| Overall survival (OS), median (95% CI), months |
6.8 (4.3, 11.6) |
4.9 (3.4, 6.8) |
6.3 (4.2, NA) |
11.6 (4.8, 16.6) |
5.6 (3.7, 8.9) |
4.2 (2.6, 5.8) |
11.6 (4.8, 16.6) |
| Overall survival for subset with WBC ≤10,000/mm3 at screening, median (95% CI), month |
6.8 (4.3, 11.6) |
5.8 (4.2, 8.0) |
6.8 (5.9, NA) |
11.6 (4.8, 16.6) |
5.8 (3.7, 8.9) |
4.3 (2.3, 6.8) |
11.6 (4.8, 16.6) |
NA: not available
Of the 14 pancreatic cancer patients evaluable for efficacy, 12 (86%) had received ≥ 2 prior lines of therapy and 10 (71%) were treated at the RD; although no objective responses were observed, 43% (n=6) of patients had disease stabilization. The median PFS among the 14 pancreatic cancer patients was 2.4 months, and median OS was 4.9 months. For the 6 patients who achieved SD as best response, the median PFS and OS were 5.0 and 6.3 months, respectively. For a pre-specified subset of pancreatic cancer patients with WBC ≤ 10,000/mm3 at screening (n=11) median OS was 5.8 months. Median OS for all 24 pancreatic cancer patients was 4.2 months.
Of the 12 colorectal cancer patients evaluable for efficacy, 9 (75%) had received ≥ 2 prior lines of therapy and 8 (67%) were treated at the RD. Although 2 patients achieved transient disease control (unconfirmed PR + stable disease), the median PFS was 1.4 months and median OS was 11.6 months. Median OS for all 14 colorectal cancer patients was also 11.6 months.
Pharmacodynamic assessment of the immune microenvironment
Paired biopsies, pre-treatment and in cycle 2, were obtained from 4 patients each with colorectal or pancreatic cancer. The paired biopsies from pancreatic cancer patients showed increased CD8+ T-cell infiltration in two patients (Supplementary Figure 2) with PFS and similar OS of 5.8 and 8.0 months, respectively, and decreased or unchanged CD8+ T-cell infiltration in the other two patients with shorter PFS/OS of 1.3/4.3 and 2.0/2.3 months, respectively. Similarly, paired biopsies showed increased CD8+ T-cell infiltration in two colorectal cancer patients with OS >4.7 and 11.8 months, respectively. For the other two colorectal cancer patients, tissue sample quantity and/or staining were insufficient on the second biopsy, so that CD8+ T-cell information could not be obtained. PD-L1 staining was negative or unchanged in 7 patients but staining was increased in one pancreatic cancer patient with OS of 5.8 months. The increase in PD-L1 staining accompanied a concomitant increase in CD8+ T-cell infiltration. (Supplementary Table 3)
Discussion
In this study, we have determined a safe and tolerable dose for the combination of CBP501/cisplatin/nivolumab. Although 83% (29/35) of patients experienced at least one TRAE, only 9% (3/35) experienced a Grade 3 TRAE and no Grade 4-5 TRAEs were reported. Of note, TRAEs of myelosuppression, nausea and vomiting, and acute kidney injury are consistent with the expected side effects of cisplatin. TRAEs of transaminase elevations are expected effects of nivolumab. Considering preclinical pharmacology data showing that CBP501 enhances the antitumor activity of cisplatin [1], it is possible that CBP501 may also exacerbate cisplatin toxicity. Overall toxicities were largely manageable by limiting CBP501 and cisplatin to a maximum of 4 cycles, and by instituting dose holds and dose reductions as needed.
Of the 14 pancreatic cancer patients evaluable for efficacy in this study, 12 (86%) had received ≥ 2 prior lines of therapy and 10 (71%) were treated at the RD. A pre-specified analysis on the survival of patients with WBC ≤ 10,000/mm3 at screening was performed based on the finding of a statistically significant difference in the survival durations of patients with WBC ≤ 10,000/mm3 and > 10,000/mm3 in all previous clinical studies with CBP501 and cisplatin among patients with platinum resistant/refractory ovarian carcinoma, malignant pleural mesothelioma, and non-squamous non-small cell lung cancer (Supplementary Table 4). Among the 14 pancreatic cancer patients evaluable for efficacy, median OS was 4.9 months overall and 5.8 months for the subset of patients with WBC ≤10,000/mm3. Although the sample size is small, results for CBP501/cisplatin/nivolumab combination therapy in ≥3rd line pancreatic cancer compare favorably with an expected median OS of 3 months [11-17].
Although clinical trials in advanced pancreatic cancer have shown no significant activity for single-agent cisplatin [9] or checkpoint inhibitors [10], there is scientific rationale for the observed activity of CBP501/cisplatin/nivolumab combination therapy in our current study. Preclinical studies showed that CBP501 enhanced cisplatin-induced DNA damage, enhanced the anti-tumor activity of cisplatin, and increased tumor immunogenic cell death that was not induced by cisplatin alone [2]. Accordingly, CBP501 in combination with cisplatin was expected to enhance the activity of checkpoint inhibitors, including monoclonal antibodies against CTLA-4, PD-1, and PD-L1. In the CT26 syngeneic tumor model, CBP501 alone, cisplatin alone, and anti-PD-1 antibody alone showed limited anti-tumor activity but the combination of CBP501/cisplatin/anti-PD-1 antibody showed strong anti-tumor activity with tumor eradication confirmed in approximately 17% of treated mice [2]. Consistent with preclinical predictions, pharmacodynamic analyses showed increased CD8+ T-cell infiltration in tumors from pancreatic cancer patients with longer PFS and OS and from colorectal cancer patients with longer OS.
Pancreatic cancer is estimated to claim 50,550 lives in the USA in 2023 [18] and options for patients who have received first or second-line therapy are slim. Based on the clinical and biological results observed in this trial, we have initiated an open-label, multicenter randomized phase 2 study to assess the efficacy and tolerance of CBP501/cisplatin/nivolumab combination therapy as 3rd-line treatment for patients with advanced exocrine pancreatic adenocarcinoma and WBC < 10,000/mm3 at screening (NCT04953962). This trial includes further dose optimization of the triplet and will evaluate the individual contributions of the addition of CBP501 and nivolumab to cisplatin.
Additional Information
Clinical Trial Registration: NCT03113188
Acknowledgements
We would like to thank the participating patients and their families, all study co-investigators and research coordinators. Medical editorial assistance was provided by Kay Noel, PhD, and was funded by CanBas Co., Ltd.
Author Contributions
, DWK, DDVH and GIS were involved in the conception and design of the study. MM, FT, SS, EHB, JE, JCM, KD, LD, SS, VK, TK and GIS were involved in the acquisition of the data. MM, FT, TK, DWK, DDVH and GIS contributed to the analysis and interpretation of the data and were involved in the review and/or revision of the manuscript. All authors approved the final manuscript and are accountable for all aspects of the work.
Ethics approval and consent to participate
The study protocol was approved by an independent institutional review board (IRB) at each investigational site: Dana-Farber Cancer Institute IRB, Ochsner Clinic Foundation IRB, and Western International Review Board on behalf of HonorHealth Research Institute. The study was conducted according to the principles of the Declaration of Helsinki and was performed in compliance with Good Clinical Practice guidelines. Written informed consent was obtained from each patient.
Data availability: The datasets generated and/or analyzed for this publication are available from the study sponsor, CanBas Co., Ltd., on reasonable request (takumi@canbas.co.jp).
Competing interests: (DRAFT before confirming by FDF) TK is an employee, patent holder on CBP501 and shareholder of CanBas Co., Ltd. DWK and DDVH are paid consultants and Scientific Advisory Board members of CanBas Co., Ltd. VK is an employee (and shareholder?) of Parexel International, which received commercial research support from CanBas Co. Ltd. GIS has received research funding from Merck KGaA/EMD-Serono, Tano Therapeutics, Bristol-Myers Squibb, Merck & Co., Pfizer and Eli Lilly. He has served on advisory boards for Merck KGaA/EMD Serono, Bicycle Therapeutics, Cybrexa Therapeutics, Bayer, Boehringer Ingelheim, ImmunoMet, Artios, Concarlo Holdings, Syros, Zentalis, CytomX Therapeutics, Blueprint Medicines, Kymera Therapeutics, Janssen and XinThera. He holds a patent entitled “Dosage regimen for sapacitabine and seliciclib” with Cyclacel Therapeutics and a patent entitled, “Compositions and Methods for Predicting Response and Resistance to CDK4/6 inhibition,” together with Liam Cornell. MM, FT, SS, EHB, JCM, KD, LD, VK reported no potential competing interests.
Funding information: This study was supported by CanBas Co., Ltd.
Supplementary information: The online version contains supplementary material available at www.britishjournalofcancerresearch.com/189suppl.pdf
References
1. Mine N, Yamamoto S, Saito N, Yamazaki S, Suda C, Ishigaki N, et al. CBP501-calmodulin binding contributes to sensitizing tumor cells to cisplatin and bleomycin. Mol Cancer Ther. 2011; 10: 1929-1938.
2. Sakakibara K, Sato T, Kufe DW, Von Hoff DD, Kawabe T. CBP501 induces immunogenic tumor cell death and CD8 T cell infiltration in combination with platinum and increases the efficacy of immune checkpoint inhibitors against tumors in mice. Oncotarget. 2017; 8: 78277-78288.
3. Mine N, Yamamoto S, Saito N, Sato T, Sakakibara K, Kufe DW, et al. CBP501 suppresses macrophage induced cancer stem cell like features and metastases. Oncotarget 8, 64015-64031 (2017)
4. Saito N, Mine N, Kufe DW, VonHoff DD, Kawabe T. CBP501 inhibits EGF-dependent cell migration, invasion and epithelial-to-mesenchymal transition of non-small cell lung cancer cells by blocking KRas to calmodulin binding. Oncotarget. 2017; 8: 74006-74018.
5. Shapiro GI, Tibes R, Gordon MS, Wong BY, Eder JP, Borad MJ, et al. Phase I studies of CBP501, a G2 checkpoint abrogator, as monotherapy and in combination with cisplatin in patients with advanced solid tumors. Clin Cancer Res. 2011; 17: 3431-3442.
6. Krug LM, Wozniak AJ, Kindler HL, Feld R, Koczywas M, Morero JZ, et al. Randomized phase II trial of pemetrexed/cisplatin with or without CBP501 in patients with advanced malignant pleural mesothelioma. Lung Cancer. 2014; 85: 429-434.
7. Paz-Ares L, Ciuleanu TE, Cobo M, Schenker M, Zurawski B, Menezes J, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncology. 2021; 22: 198-211.
8. Garassino MC, Gadgeel S, Speranza G, Felip E, Esteban E, Dómine M, et al. Pembrolizumab Plus Pemetrexed and Platinum in Nonsquamous Non-Small-Cell Lung Cancer: 5-Year Outcomes From the Phase 3 KEYNOTE-189 Study. J Clin Oncol. 2023;41(11):1992-1998.
9. Gresham GK, Wells GA, Gill S, Cameron C, Jonker DJ. Chemotherapy regimens for advanced pancreatic cancer: a systematic review and network meta-analysis. BMC Cancer. 2014; 14: 471.
10. Macherla S, Laks S, Naqash AR, Bulumulle A, Zervos E, Muzaffar M. Emerging role of immune checkpoint blockade in pancreatic cancer. Int J Mol Sci. 2018; 19: 3505.
11. Manax, V.G., , Chatikhine, V., Kirven, S., Taylor, B.R. Designing clinical trials in 3L+ pancreatic cancer. JCO 37, Meeting Abstract | 2019 Gastrointestinal Cancers Symposium.
12. Wang-Gillam A, Li CP, Bodoky G, Dean A, Shan YS, Jameson G, et al. NAPOLI-1 Study Group. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomized, open-label, phase 3 trial. Lancet. 2016; 387: 545-557.
13. Assaf E, Verlinde-Caravalho M, Debaldo C, Grenier J, Sellam Z, Pouessel D, et al. 5-Fluorouracil/leucovorin combined with irinotecan and oxaliplatin (FOLFIRINOX) as second-line chemotherapy in patients with metastatic pancreatic adenocarcinoma. Oncology. 2011; 80: 301-306.
14. Lee MG, Lee SH, Lee SJ, Lee YS, Hwang JH, Ryu JK, et al. 5-Fluorouracil/leucovorin combined with irinotecan and oxaliplatin (FOLFIRINOX) as second-line chemotherapy in patients with advanced pancreatic cancer who have progressed on gemcitabine-based therapy. Chemotherapy. 2013; 59: 273-279.
15. Bodoky G, Timcheva C, Spigel DR, La Stella PJ, Ciuleanu TE, Tebbutt NC. A phase II open-label randomized study to assess the efficacy and safety of selumetinib (AZD6244[ARRY-142886]) versus capecitabine in patients with advanced or metastatic pancreatic cancer who have failed first-line gemcitabine therapy. Invest New Drugs. 2012; 30: 1216-1223.
16. Hurwitz H, Uppal N, Wagner SA, Bendell JC, Beck JT, Wade SM 3rd, et al. Randomized, double-blind, phase II study of ruxolitinib or placebo in combination with capecitabine in patients with metastatic pancreatic cancer for whom therapy with gemcitabine has failed. J Clin Oncol. 2015; 33: 4029-4047.
17. Seery TE, Nangia CS, McKean HA, Sender LS, Bhar P, Reddy SK, et al. Promising survival and disease control in third-line or greater metastatic or locally advanced pancreatic cancer patients following chemo-radiation and novel combination of aldoxorubicin, N-803 IL-15 superagonist, and PDL1- NK cell therapy. JCO. 2022; 40 (Suppl 4): (abstract 582).
18. Siegel RL, Miller KD, Wagle Jemal A. Cancer statistics. 2023; CA: Cancer J Clinicians (https://doi.org/10.3322/caac.21763)
Received: January 5, 2023;
Accepted: February 13, 2023;
Published: February 19, 2023.
To cite this article : Matrana M, Tsai F, Satti S, Borazanci EH, Moser JC, Do K, et al. Clinical Significance of Serum miR-21, CA153 and CEA in Breast Cancer. British Journal of Cancer Research. 2024; 7(1): 648- 655. doi: 10.31488/bjcr.189.
© The Author(s) 2024. This is an open access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/).
