Research article / Open Access
DOI: 10.31488/bjcr.179
A Review of the COBRA Protocol and its Clinical Importance
Walaa K. Aldhahri, MBBS1,2, Akshar V. Kalola, DO1,2, Claire A. Valburg, MD1,2, Alexandra Zara Rozalen, MD3, Ramesh Subrahmanyam, PhD2, Victor E. Nava, MD, PhD3, Maneesh Jain, MD2
1. Department of Hematology and Oncology, George Washington University Hospital, Washington, DC, 20037
2.Department of Hematology and Oncology, The Edward P. Evans Foundation Center of Excellence for Prostate Cancer, Washington DC VA Medical Center, Washington, DC 20422
3. Department of Pathology, Washington DC VA Medical Center, Washington, DC 20422
*Corresponding author:Maneesh Jain, Department of Hematology and Oncology, Washington DC VA Medical
Center, Washington, DC, USA
All authors contributed equally to this manuscript
Introduction
Prostate cancer is the most common non-skin cancer in the USA among men and a leading cause of cancer morbidity worldwide. The majority of prostate cancers present as localized disease, but metastatic disease remains a significant cause of morbidity and mortality [1].
While localized, low-risk prostate cancers may be treated with non-systemic therapies including radiation and/or surgery, metastatic disease requires the addition of systemic therapy in the form of androgen deprivation therapy [2, 3]. Although androgen deprivation therapy (ADT) is usually effective in curbing the initial progression of cancer, after time, it often becomes ineffective, and the disease progresses to metastatic castration resistant prostate cancer (mCRPC) [4].
The current FDA approved therapies for mCRPC reflect the different pathways in which mCRPC is thought to develop. Such medications include second-generation androgen receptor (AR) targeting agents as well as medications that target tumors containing alterations in DNA repair genes. It is believed that about 60% of patients with advanced prostate cancer have molecular alterations in pathways unrelated to androgen receptor signaling. These mutations are thought to occur mainly in genes that encode components of the DNA damage response (DDR), such as BRCA1 and BRCA2, making the cancers feasible targets of DNA damaging therapy [5].
Given the complexity of DNA replication, there are different DNA repair pathways according to the type of DNA damage, but most are governed by a set of general enzymatic roles. The DDR machinery roles include damage sensors, transducer kinases, and effectors which maintain genomic stability and monitor the accurate transmission of genetic information. The repair pathways share similarities, but, in general, a damage sensor recognizes specific DNA damage before recruiting and activating downstream transducer kinases (such as ATM, ATR, DNA-PKcs), which in turn transduce the signal to effector proteins (such as BRCA1 and BRCA2) [6]. When both DNA strands are damaged, the main systems involved in the repairing process are non-homologous end joining (NHEJ) and homologous recombination repair (HRR). On the other hand, if damage occurs only in one strand, the unaltered strand works as a framework for mismatch repair (MMR), nucleotide excision repair (NER), base excision repair (BER), and single-strand break repair pathways (SSBR) [7].
While it is hypothesized that patients with genetic mutations in DDR pathways may respond well to DNA damaging therapies, to date, trials on the prognosis of advanced prostate cancer patients with DDR gene aberrations and the associated response to therapies are limited [8]. Some studies have shown a high prevalence of germline mutations in DDR genes and, for that reason, the National Comprehensive Cancer Network (NCCN) recommends germline testing for all men with high-risk localized prostate cancer and those with metastatic disease [9]. Current analyses have revealed that approximately 33% of mCRPC tumors contain biallelic inactivation of BRCA1, BRCA2 or ATM [10]. Most of the mutations are found primarily in BRCA2, but other genes have also been identified [11, 12].
The mutations in DDR genes would seemingly make DNA damaging therapies attractive treatment options and potential avenues for future research. Poly-ADP-ribose polymerase (PARP) inhibitors are one such DNA damaging treatment option. To date, two PARP inhibitors have been approved by the FDA for use in prostate cancer in patients with mutations in DDR genes: rucaparib and olaparib [13, 14]. The latter of these, olaparib, is effective for tumors with mutations in a specific subset of DDR genes, so called HRR deficient tumors. In the PROfound study, patients with HRR mutations treated with olaparib were found to have improved progression free survival (PFS) times when compared to the control [15].
Other such targeted DNA damaging therapies include platinum-based therapies. Although research in this area is limited, it has been reported that DNA repair defects may be predictors of sensitivity to platinum agents, suggesting its use as targeted therapy in mCRPC [16-19]. One platinum agent, carboplatin, has demonstrated some clinical activity against mCRPC in numerous phase II trials and studies: carboplatin, given with or after chemotherapy, resulted in improved PFS and clinical and biochemical response [20-22]. Platinum-based therapy has been successful in treating breast and ovarian cancers with pathogenic mutations in BRCA1 or BRCA2. In patients with mCRPC with biallelic mutations in BRCA2, a significant PSA reduction was observed after carboplatin treatment, raising the possibility that biallelic BRCA2 inactivation may function as a predictive biomarker for sensitivity to platinum-based therapy in mCRPC [18].
As noted above, a significant proportion of metastatic prostate cancers involve mutations in the HRR pathway. While olaparib and carboplatin are thought to be beneficial for patients with mutated HRR genes, at this time, prospective trials for side-by-side comparison of these targeted therapies to explore efficacy or synergistic effects after synchronous or successive use are lacking.
The purpose of the COBRA (Carboplatin or olaparib for BRCA Deficient Prostate Cancer) Protocol is to answer these questions and establish if there is a better response to carboplatin compared to olaparib in patients with HRR deficient tumors.
Methods
The COBRA Protocol is a phase II, unblinded, multi-center, randomized crossover study with a primary endpoint that assesses the efficacy of carboplatin versus olaparib as first-line therapy in the treatment of mCRPC. Specifically, it will evaluate mCRPC harboring inactivating mutations in the HRR pathway (BRCA1, BRCA2, PALB2) that have been previously treated with AR blocking agents. An amendment was recently made to include new qualifying mutations: BARD1, BRIP1, CHEK1, FANCL, RAD51B, RAD51C, RAD51D, and RAD54L. The primary endpoint is the time between randomization of the patient into a treatment group and disease progression as defined by radiographic disease progression or measurable disease by the RECIST 1.1 guidelines or death due to any cause [23]. Upon disease progression, the patient will be started on the other medication and the crossover portion of the COBRA Protocol will begin. The second-line medication will be continued until there is second documented disease progression (defined as radiographic or measurable disease progression by RECIST 1.1 guidelines) or death due to any cause during or after the second line treatment and the study will be terminated as demonstrated in figure 1.
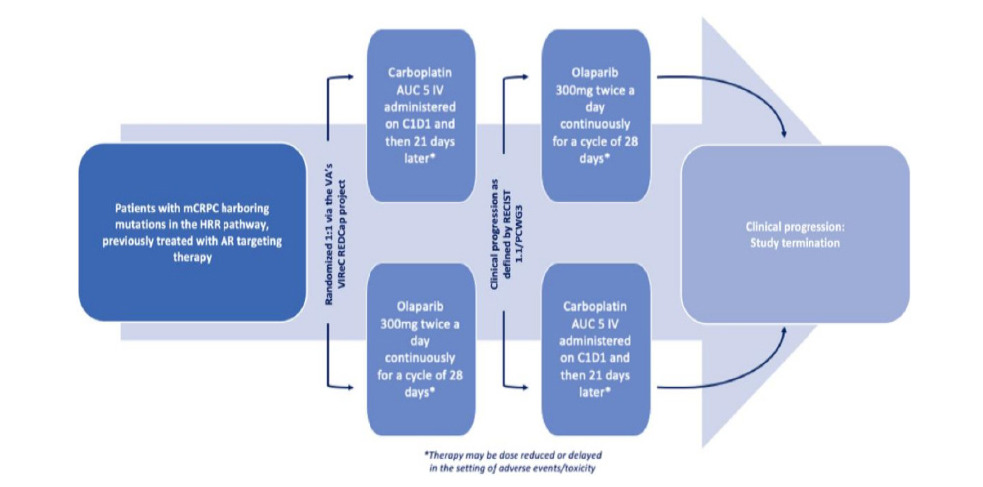
Figure 1.COBRA Protocol treatment schematic
The data collected during and after the crossover phase informs much of the secondary objectives of the Protocol. These objectives include assessment of combined PFS time between initial randomization and disease progression on or after medication of the second arm of treatment; PFS time between the start of the second line treatment (after progression of disease on the initial treatment arm) and disease progression or death due to any cause; PSA response and duration of response; and the Grade 3 and 4 toxicities of both carboplatin and olaparib. The percentage of patients obtaining a 50% and 90% reduction, respectively, in PSA according to PCWG3 criteria, as well as a 30% reduction of measurable disease by RECIST 1.1 guidelines will also be measured [23, 38].
For first line treatment, the first treatment arm will receive carboplatin AUC 5 IV administered every 21 days; the second treatment arm will receive olaparib 300 mg twice a day continuously in 28-day cycles. All participants will have to undergo the informed consent procedure and must sign the informed consent and HIPAA authorization prior to participating in the clinical trial. Screening procedures including imaging (e.g. CT, MRI, or bone scan) are to be completed during the screening period ≤ 30 days prior to initiation of treatment. Imaging will then be repeated every 12 weeks ± 7 days or as clinically indicated to evaluate for progression of disease. Upon objective evidence of disease progression, patients will be transitioned to the second line of therapy (carboplatin to olaparib or olaparib to carboplatin) after a minimum washout period of 2 weeks. Labs, imaging, and physical examination will be repeated prior to crossover to second- line therapy and then again at termination of the study (when possible).
To be a candidate for the Protocol, rigorous inclusion and exclusion criteria guidelines were enacted to ensure the validity of the study and the safety of the patients (Table 1).
Table 1.Inclusion and exclusion criteria outlined in the COBRA protocol
| Inclusion criteria | Exclusion criteria |
|---|---|
| Males age >18 years, with signed informed consent form and HIPAA authorization form | Currently enrolled in another investigational drug or device study or receiving treatment for other neoplasms |
| ECOG performance status <2 | Histologic evidence of pure small-cell or high-grade neuroendocrine tumor |
| Diagnosis of prostate cancer (excluding pure small-cell or pure high-grade neuroendocrine histology) | Having received investigational therapeutics within 30 days of the study |
| Ongoing androgen deprivation therapy | Having been treated previously with platinum agents, PARP inhibitors, or mitoxantrone for mCRPC |
| Metastatic disease as defined as measurable disease progression based on RECIST 1.1 criteria, radiographic progression of disease on bone scan, CT scan, or MRI with >2 lesions, or PSA level >2 ng/ml that has risen on 2 successive occasions at least 1 week apart in the setting of serum testosterone < 50 ng/ml | Concomitantly using strong CYP3A inducers or inhibitors without the appropriate washout period prior to the study |
| Prior therapy with abiraterone acetate, enzalutamide, apalutamide, or darolutamide | Gastrointestinal/swallowing disorders that interfere with the ability of the study medications to be appropriately absorbed |
| Confirmed mutations in BRCA1, BRCA2, PALB2, BARD1, BRIP1, CHEK1, FANCL, RAD51B, RAD51C, RAD51D, and RAD54L as assessed by a CLIA certified assay | Parenchymal brain metastasis |
| Normal bone marrow function as defined by an absolute neutrophil count (ANC) > 1.5 x 109/L and platelet count > 100 x 109/L | Myelodysplastic syndrome/AML (or with features suggestive of such) |
| Normal hepatic function defined as total bilirubin < 1.5 times the institutional upper limit of normal (ULN), AST and ALT < 2.5 times the institutional ULN unless liver metastases are present in which case, they must be < 5 times the ULN | Clinically significant heart disease (e.g. recent MI or arterial thrombotic event, New York Health Association (NYHA) Class II-IV heart disease, EF <35%, severe angina, unstable angina, etc.) |
| Normal renal function as defined by creatinine clearance (CrCl) of > 30 mL/min (in the case of CrCl 31-50 mL/min olaparib dosage adjustments will be necessary) | Other psychiatric, neurologic, or social conditions which interfere with their ability to give consent or follow up as directed |
Patients are being recruited from multiple Veterans Affairs (VA) oncology clinics throughout the US. Patients are pre-screened for a diagnosis of mCRPC through medical records; their medical history is also screened to evaluate for previous treatment with first-line therapy, as well as BRCA1, BRCA2, PALB2, BARD1, BRIP1, CHEK1, FANCL, RAD51B, RAD51C, RAD51D, and/or RAD54L. Approximately 25 patients will be enrolled in the study per year over four years for a total enrollment of about 100 patients.
To ensure appropriate randomization, patients are first stratified. This is being done based on two conditions: the presence or absence of visceral (lung or liver) involvement and the prior receipt of docetaxel. The patients are then randomized through the VA’s VIReC Research Electronic Data Capture (REDCap) project to either of the treatment arms.
Both carboplatin and olaparib do have adverse effects and safety concerns. Carboplatin, while better tolerated than cisplatin, still has a number of side effects including bone marrow suppression, gastrointestinal toxicity and hepatic toxicity. Olaparib’s side effects seen in the PROfound trial, occurred in > 10% of patients, and included anemia, fatigue, nausea, decreased appetite, diarrhea, vomiting, thrombocytopenia, cough, and dyspnea [46, 47]. Additionally, olaparib is metabolized by the CYPY3A system and numerous medications may affect its metabolism.
As patient safety is paramount, protocol deviations including dose reductions and treatment delays are allowed in the setting of NCI CTCAE Grade 3-4 toxicity (and Grade 1-2 if deemed significant by the investigator). Treatment delays are allowed for a maximum of 6 weeks before patients are withdrawn from the study.
Additionally, patient safety and tolerability are monitored by evaluation for adverse events (AE) (graded per the NCI CTCAE v 5.0), participation in regular physical examinations, and collection of vital signs and labs. All AEs of Grade 3-4 per NCI CTCAE are recorded and reported to the VA central IRB both verbally and in writing. Regular lab, imaging, and physical examinations occur during study treatment, and the evaluation of patient eligibility is reassessed at every follow up clinic visit. Grade 3 or above AEs are followed and managed after study termination until their resolution or they are determined to be chronic conditions. The safety and tolerability of the study drugs will be analyzed according to the secondary objectives.
Discussion
As noted at the outset, prostate cancer and, especially, mCRPC remain a significant cause of morbidity and mortality in men across the world. Since mCRPC is not a singular disease caused by a distinct process, but rather a constellation of processes caused by a few or numerous mutations in the genome, treatment plans should ideally be targeted to the tumor's unique genetic landscape to maximize efficiency and the chance of survival in the patient. Such a belief has driven genomics and cancer research in this area. However, due to the poor prognosis associated with mCRPC, it is incredibly important to continue to develop and test targeted treatment options for this population. The goal of the COBRA Protocol is to develop improved treatment standards for patients with HRR mutations.
As of today, the mainstays of treatment for mCRPC include AR targeting agents abiraterone or enzalutamide, radium 223, Sipuleucel-T and taxanes [24]. Taxanes like docetaxel and cabazitaxel have demonstrated good initial response and survival benefit. Unfortunately, almost all mCRPC become resistant to taxanes and eventually continue to progress [25]. Because of this, targeted avenues of therapy have been proposed and developed to help slow or stop progression of disease. One such targeted approach has taken the form of DNA damaging therapies, like platinum-based therapy and PARP inhibitors which may play a role in HRR mutated tumors.
HRR deficiency can result from mutations in BRCA1, BRCA2, ATM, RAD51D, PALB2, BARD1, BRIP1, CHEK1, FANCL, RAD51B, RAD51C, RAD51D, and RAD54L genes which disrupt the DNA repair pathway. Prostate tumor testing revealed that approximately 11% of all metastatic prostate cancers contained germline DNA repair gene mutations while it has been noted to be as high as 33% in mCRPC [26, 27]. The significant prevalence of these mutations and the availability of targeting drugs makes prostatic carcinoma trials a promising area for successful advancement in oncologic therapy. For this reason, Carboplatin and olaparib were chosen for the COBRA trial.
Platinum-based therapy has been used to treat numerous cancers. Carboplatin, specifically, has been used in the treatment of ovarian, head, neck, breast, and lung cancers. Recently, with increased genomic sequencing and analysis, the impact of certain DNA repair mutations on the sensitivity and response of a tumor to platinum-based therapy has been assessed. The importance of BRCA1 and BRCA2 for DNA double-strand break repair by HRR is undeniable. HRR-mutated cancers of various types have demonstrated increased sensitivity to platinum-based therapy. For example, ovarian, fallopian tube, and peritoneal carcinomas with germline or somatic HRR mutations treated with platinum therapy were found to have improved response and survival [28].
In addition, a significant body of research supports the importance of BRCA1 and BRCA2 in breast cancer outcomes and responses to treatment. A phase III trial comparing docetaxel vs carboplatin found that patients with germline BRCA1 and BRCA2 mutations had a better response and outcome to carboplatin than to docetaxel. The outcomes of the patients with BRCA1 and BRCA2 mutations were then compared to those without the mutations, and improved treatment responses to carboplatin were not observed for unmutated phenotypes [29]. This study highlighted the sensitivity of BRCA1 and BRCA2 mutated breast cancer to carboplatin, suggesting its potential use in other tumors containing BRCA1 and BRCA2 mutations.
A number of clinical trials exploring the use of platinum-based therapy for mCRPC have demonstrated clinical activity of carboplatin against mCRPC [20, 21, 30, 31, 32]. The combination of carboplatin plus paclitaxel was evaluated in 38 patients, of whom 24 had already received two or more prior chemotherapy regimens. There was a clinical and/or biochemical response in 26% of cases, and an additional 34% demonstrated stable disease. When patients were being treated with the biweekly regimen of paclitaxel and carboplatin, the median duration of response and median time to progression were 6 and 3.6 months respectively, and the median overall survival was 10 months [21]. In another trial, the combination of carboplatin plus docetaxel was given to 34 men on or within 45 days of completion of docetaxel chemotherapy. Biochemical response was observed in 18% of cases, median PFS was 3 months, and median overall survival was 12 months [22].
Some studies have shown the potential of platinum-based compounds as a future targeted therapy in prostate cancer [19]. While many of the smaller-scale studies starting in the 1980s showed limited benefit with platinum-based therapy, they were limited by the lack of technological capability in detecting mutations that might affect response to such therapy [33, 34]. Since then, there has been evidence via several preclinical studies of an improved response to platinum-based therapy if the prostate cancer had mutations in HRR DNA repair genes or DNA damage checkpoints, including BRCA1, BRCA2, PALB2, NSB1, CHK2 [35, 36]. In one study, 141 men with mCRPC were treated with carboplatin and docetaxel, showing a significant improvement in patients with germline BRCA2 mutations [37]. Interestingly, 6 out of 8 patients with taxane refractory mCRPC who were BRCA2 carriers demonstrated a 50% decline in PSA with the addition of carboplatin, and their survival was twice that of patients who were not carriers.
A study conducted at the Memorial Sloan Kettering Cancer Center in New York echoed these results. The study evaluated the efficacy of platinum-based therapy in DDR mutant mCRPC [39]. The DDR mutations were found in 16 of 64 patients (25%) and were associated with an increased probability of achieving a PSA decline of 50% or more from baseline. Of 8 patients with DDR mutated mCRPC who received platinum-based therapy after a PARP inhibitor, 3 out of 7 evaluable patients had radiographic partial response or stable disease, and 2 out of 7 had a PSA reduction response.
In addition to platinum-based therapies, BRCA mutated cancers have been approved for treatment with PARP inhibitors. With regard to ovarian cancer, olaparib was the first PARP inhibitor to be approved for treatment given the positive results in the SOLO1, PRIMA and PAOLA-1 phase III trials [40]. In the OlympiAD trial, olaparib demonstrated improved PFS versus conventional chemotherapy in patients with metastatic HER2-negative BRCA mutated breast cancer [41]. With regard to pancreatic cancer, the POLO trial demonstrated improved outcomes of olaparib versus placebo in pancreatic cancers with germline BRCA1 and BRCA2 mutations [42,43]. At this time, olaparib has been FDA approved in the treatment of breast, ovarian, fallopian tube or primary peritoneal cancer, pancreatic, and prostate cancer.
In the PROfound study, a randomized phase III trial, PFS in the group with BRCA1, BRCA2, and/or ATM mutations treated with olaparib was 7.4 months vs. 3.6 months in the control (P < 0.001) and secondary endpoints also favored those receiving olaparib [15]. While the study showed significant differences when compared against ADT and hormonal therapy, it was limited in assessing the difference between targeted therapies.
A similar drug, rucaparib, was studied specifically with regard to mCRPC. In the TRITON2 study, a phase II single arm model, patients with prior-treated mCRPC with confirmed BRCA1 or BRCA2 mutations were given rucaparib. The result of the study showed a significant decrease in PSA levels (≥ 50% decrease from baseline) in 54.8% of patients [44]. In 2020, both the PARP inhibitors olaparib and rucaparib were FDA approved for treatment of patients mCRPC with genomic alterations involving HRR genes [35].
The data and conclusions of previous treatment trials have laid the foundation of the COBRA Protocol. The emerging positive results using PARP inhibitors and platinum-based therapy for cancers with DDR are promising and require additional trials to further optimize these treatment options. The COBRA Protocol aims to do precisely that in a prospective clinical trial assessing carboplatin and olaparib as first line treatments for specific subsets of mCRPC. Interestingly, both therapies are noted to operate on similar mutation domains and may provide the opportunity to explore synergy. The COBRA Protocol, unlike trials in the past, will be a prospective study to evaluate responses of patients who have HRR deficient tumors. Hopefully, this protocol will help to optimize response, and it will reduce statistical variance and noise to allow for a more accurate assessment of each medication’s potential to increase PFS.
Furthermore, the study is designed not only to compare each medication’s impact on the patient, but also to determine if there is any benefit to using both medications. In the crossover portion, patients will be exposed to the other treatment. The responses of the patients and the progression (or lack thereof) of mCRPC may help shed light on the pathogenesis of such cancers. Should the patients experience a synergistic effect and improve more than expected after both medications, this research may point to combining these medications as first line treatment. The outcome data generated by this protocol will hopefully be beneficial not only to the enrolled patients but also to the healthcare economy.
The COBRA trial will be conducted with a consortium of prostate cancer groups involving multiple Veterans Affairs Medical Centers in a variety of locations throughout the United States with approval from the VA Central IRB. Such variety in academic sites and geography will help mirror the populations in prior studies, as well as, providing economic, ethnic, and racial diversity to be more reflective of the overall population of prostate cancer patients.
Because personalized therapies for prostate cancer progression are lacking, different efforts to better understand the disease and its resistance to treatment are necessary to find new therapeutic approaches. Genomic analysis allows identification of specific pathways for targeted treatment, and the use of combination therapy with different mechanisms of action constitutes a promising approach to overcome resistance to treatment. Efforts continue to better explore the molecular alterations that lead to prostate cancer at different stages of the disease and to identify and validate predictors of response to treatment. The COBRA Protocol and the data collected will hopefully contribute to these goals and drive future research providing patients with mCRPC opportunities for an improved prognosis.
References
1. American Cancer Society. Facts & Figures 2022. American Cancer Society. Atlanta, GA. 2022
2. InformedHealth.org [Internet]. Cologne, Germany: Institute for Quality and Efficiency in Health Care (IQWiG); 2006-Updated 2020. Localized prostate cancer: Low-risk prostate cancer: Active surveillance or treatment?. Available from: https://www.ncbi.nlm.nih.gov/books/NBK487255/
3. National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology. Prostate cancer Version 4.2022. 2022;National Comprehensive Cancer Network. Available from: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf
4. Yang X, Chen H, Xu D, et al. Efficacy and safety of Androgen Deprivation Therapy (ADT) combined with modified docetaxel chemotherapy versus ADT combined with standard docetaxel chemotherapy in patients with metastatic castration-resistant prostate cancer: study protocol for a multicentre prospective randomized controlled trial. BMC Cancer. 2022;22(1):177. doi: 10.1186/s12885-022-09276-y.
5. Robinson D, Van Allen, E M, et al. Integrative clinical genomics of advanced prostate cancer. Cell. 2015; 161:1215–1228.
6. Hitomi K, Iwai S, Tainer JA. The Intricate Structural Chemistry of Base Excision Repair Machinery: Implications for DNA Damage Recognition, Removal, and Repair. DNA Repair (Amst) (2007) 6:410–28. doi: 10.1016/j.dnarep.2006.10.004.
7. Mateo J, Boysen G, Barbieri CE, et al. DNA Repair in Prostate Cancer: Biology and Clinical Implications. Eur. Urol. 2017;71:417–425. doi: 10.1016/j.eururo.2016.08.037.
8. Lozano R, Castro E, Aragón IM, et al. Genetic aberrations in DNA repair pathways: a cornerstone of precision oncology in prostate cancer. Br J Cancer. 2021;124(3):552-563. doi: 10.1038/s41416-020-01114-x.
9. Doan DK, Schmidt KT, Chau CH, et al. Germline Genetics of Prostate Cancer: Prevalence of Risk Variants and Clinical Implications for Disease Management. Cancers (Basel). 2021;13(9):2154. doi: 10.3390/cancers13092154.
10. Messina C, Cattrini C, Soldato D, et al. BRCA Mutations in Prostate Cancer: Prognostic and Predictive Implications. J Oncol. 2020;2020:4986365. doi: 10.1155/2020/4986365.
11. Armenia J, Wankowicz SAM, Liu D, et al. PCF/SU2C International Prostate Cancer Dream Team, Schultz N, Van Allen EM. The long tail of oncogenic drivers in prostate cancer. Nat Genet. 2018;50(5):645-651. doi: 10.1038/s41588-018-0078-z.
12. Nielsen FC, van Overeem Hansen T, Sørensen CS. Hereditary breast and ovarian cancer: new genes in confined pathways. Nat Rev Cancer. 2016;16(9):599-612. doi: 10.1038/nrc.2016.72.
13. Package Insert. LYNPARZA® (olaparib) tablets, for oral use. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2021.
14. Package Insert. RUBRACA® (rucaparib) tablets, for oral use. Boulder, CO: Clovis Oncol. Inc.; 2021.
15.De Bono JS, Matsubara N, Penel N, et al. Exploratory gene-by-gene analysis of olaparib in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC): PROfound. Presented at: 2021 Genitourinary Cancers Symposium. 2021. Abstract 126.
16. Pomerantz MM, Spisak S, Jia L, et al. The association between germline BRCA2 variants and sensitivity to platinum-based therapy among men with metastatic prostate cancer. Cancer 2017;123:3532- 3539
17. Schmid S, Omlin A, Higano C, et al. Activity of Platinum-based therapy in Patients With Advanced Prostate Cancer With and Without DNA Repair Gene Aberrations. JAMA Netw Open 2020;3:e2021692.
18. Cheng HH, Pritchard CC, Boyd T, et al. Biallelic Inactivation of BRCA2 in Platinum-sensitive Metastatic Castration-resistant Prostate Cancer. Eur Urol. 2016;69(6):992-5. doi: 10.1016/j.eururo.2015.11.022.
19. Hager S, Ackermann CJ, Joerger M, et al. Anti-tumour activity of platinum compounds in advanced prostate cancer-a systematic literature review. Ann Oncol 2016;27:975-984
20. Cabrespine A, Guy L, Khenifar E, et al. Randomized Phase II study comparing paclitaxel and carboplatin versus mitoxantrone in patients with hormone-refractory prostate cancer. Urology. 2006;67(2):354-9. doi: 10.1016/j.urology.2005.08.046.
21. Kentepozidis N, Soultati A, Giassas S, et al. Paclitaxel in combination with carboplatin as salvage treatment in patients with castration-resistant prostate cancer: a Hellenic oncology research group multicenter phase II study. Cancer Chemother Pharmacol. 2012;70(1):161-8. doi: 10.1007/s00280-012- 1896-9.
22. Ross RW, Beer TM, Jacobus S, et al. Prostate Cancer Clinical Trials Consortium. A phase 2 study of carboplatin plus docetaxel in men with metastatic hormone- refractory prostate cancer who are refractory to docetaxel. Cancer. 2008;112(3):521-6. doi: 10.1002/cncr.23195.
23. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228– 47.
24. Teo MY, Rathkopf DE, Kantoff P. Treatment of Advanced Prostate Cancer. Annu Rev Med. 2019;70:479-499. doi: 10.1146/annurev-med-051517-011947.
25. Ruiz de Porras V, Font A, Aytes A. Chemotherapy in metastatic castration-resistant prostate cancer: Current scenario and future perspectives. Cancer Lett. 2021;523:162-169. doi: 10.1016/j.canlet.2021.08.033.
26. Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-Repair Gene Mutations in Men with Metastatic Prostate Cancer. N Engl J Med. 2016;375(5):443-53. doi: 10.1056/NEJMoa1603144.
27. Castro E, Goh C, Olmos D, et al. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J Clin Oncol. 2013; 31:1748-57.
28. Pennington KP, Walsh T, Harrell MI, et al. Germline and somatic mutations in homologous recombination genes predict platinum response and survival in ovarian, fallopian tube, and peritoneal carcinomas. Clin Cancer Res. 2014;20(3):764-75. doi: 10.1158/1078-0432.CCR-13-2287.
29. Tutt A, Tovey H, Cheang MCU, et al. Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: the TNT Trial. Nat Med. 2018;24(5):628-637. doi: 10.1038/s41591-018-0009-7.
30. Mota JM, Barnett E, Nauseef JT, et al. Platinum-based therapy in Metastatic Prostate Cancer With DNA Repair Gene Alterations. JCO Precis Oncol. 2020;4:355- 366. doi: 10.1200/po.19.00346.
31. Leal F, García-Perdomo HA. Effectiveness of Platinum-based therapy in Patients With Metastatic Prostate Cancer: Systematic Review and Meta-analysis. Clin Genitourin Canc. 2019 17(3):e627- e644. doi: 10.1016/j.clgc.2019.03.008.
32. Nader R, El Amm J, Aragon-Ching JB. Role of chemotherapy in prostate cancer. Asian J Androl. 2018;20(3):221-229. doi: 10.4103/aja.aja_40_17.
33. Yagoda A, Petrylak D. Cytotoxic chemotherapy for advanced hormone-resistant prostate cancer. Cancer. 1993;71(3 Suppl):1098-109. doi: 10.1002/1097-0142(19930201)71:3+<1098::aid- cncr2820711432>3.0.co;2-g.
34. Qazi R, Khandekar J. Phase II study of cisplatin for metastatic prostatic carcinoma. An Eastern Cooperative Oncology Group study. Am J Clin Oncol. 1983;6(2):203-5. doi: 10.1097/00000421- 198304000-00011.
35. Yamada Y, Beltran H. The treatment landscape of metastatic prostate cancer. Cancer Lett. 2021;519:20-29. doi: 10.1016/j.canlet.2021.06.010.
36. Hager S, Ackermann CJ, Joerger M, et al. Anti-tumour activity of platinum compounds in advanced prostate cancer-a systematic literature review. Ann Oncol. 2016 Jun;27(6):975- 984. doi: 10.1093/annonc/mdw156.
37. Pomerantz MM, Spisák S, Jia L, et al. The association between germline BRCA2 variants and sensitivity to platinum-based therapy among men with metastatic prostate cancer. Cancer. 2017;123(18):3532-3539. doi: 10.1002/cncr.30808.
38. Scher HI, Morris MJ, Stadler WM, et al. Prostate Cancer Clinical Trials Working Group 3. Trial Design and Objectives for Castration-Resistant Prostate Cancer: Updated Recommendations From the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol. 2016;34(12):1402-18. doi: 10.1200/JCO.2015.64.2702.
39. Mota JM, Barnett E, Nauseef JT, et al. Platinum-based therapy in Metastatic Prostate Cancer With DNA Repair Gene Alterations. JCO Precis Oncol. 2020;4:355- 366. doi: 10.1200/po.19.00346.
40. Foo T, George A, Banerjee S. PARP inhibitors in ovarian cancer: An overview of the practice-changing trials. Genes Chromosomes Canc. 2021;60(5):385-397. doi: 10.1002/gcc.22935.
41. Robson M, Im SA, Senkus E, et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N Engl J Med. 2017;377(6):523-533. doi: 10.1056/NEJMoa1706450.
42. Park W, Chawla A, O'Reilly EM. Pancreatic Cancer: A Review. JAMA. 2021;326(9):851-862. doi: 10.1001/jama.2021.13027.
43. Golan T, Hammel P, Reni M, et al. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N Engl J Med. 2019;381(4):317-327. doi: 10.1056/NEJMoa1903387.
44. Abida W, Patnaik A, Campbell D, et al. TRITON2 investigators. Rucaparib in Men With Metastatic Castration- Resistant Prostate Cancer Harboring a BRCA1 or BRCA2 Gene Alteration. J Clin Oncol. 2020;38(32):3763-3772. doi: 10.1200/JCO.20.01035.
45. Berchuck JE, Silver R, Bakouny Z, et al. Response to olaparib or carboplatin in a real-world cohort of men with DNA damage repair (DDR) deficient metastatic castration-resistant prostate cancer (mcrpc). J Clin Oncol. 2020;38(6_suppl):43-43. doi:10.1200/jco.2020.38.6_suppl.43
46. de Bono J, Mateo J, Fizazi K, et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2020;382(22):2091-2102. doi: 10.1056/NEJMoa1911440.
47. Hussain M, Mateo J, Fizazi K, et al. PROfound Trial Investigators. Survival with Olaparib in Metastatic Castration- Resistant Prostate Cancer. N Engl J Med. 2020;383(24):2345-2357. doi: 10.1056/NEJMoa2022485.
Received: November 07, 2022;
Accepted: December 01, 2022;
Published: December 05, 2022.
To cite this article : Aldhahri W, Kalola A, Valburg CA, et al. A Review of the COBRA Protocol and its Clinical Importance. British Journal of Cancer Research. 2022; 5(2): 584- 590. doi: 10.31488/bjcr.179.
©2022 Aldhahri W, et al.
