Research article / Open Access
DOI: 10.31488/bjcr.181
Application of Thermography and Neural Network Classification for Early Detection of Breast Cancer
Rubens Fernando de Lima Mendrone1, Alysson Fernandes Mazoni2,3, Jaime Manuel Ricardo do Espírito Santo, Nirali Rathwa4, Raphael Brandão Moreira5, Daniella Zanetti Bucci*6
1.Linda Canada Artificial Intelligence Inc. 88 Queens Quay West Suite 2500, Toronto, ON, M5J 0B8, Canada
2. Buildbox IT Solutions. Rua Augusto César de Andrade, 1567. Bairro Nova Campinas. Campinas, São Paulo. CEP: 13092- 117, Brazil
3. Institute of Geosciences - University of Campinas. Rua Carlos Gomes, 250, Cidade Universitária, Campinas, São Paulo. CEP 13083-855, Brazil
4. MaRS Discovery District. MaRS Centre, South Tower, 101 College Street, Suite 100, Toronto, ON MG5 1L7, Canada
5. Clinica FIRST. Praça Pereira Coutinho, 83, Vila Nova Conceição, São Paulo. CEP 04510-010, Brazil
6. Termo Health Tecnologia S/A. Avenida Marcos Penteado de Ulhoa Rodrigues, 939, torre Jacarandá, 8º andar, sala 873. Bairro Tamboré. Barueri, São Paulo. CEP: 06460-040, Brazil
*Corresponding author:Daniella Zanetti Bucci, Termo Health Tecnologia S/A. Avenida Marcos Penteado de Ulhoa Rodrigues, 939, torre Jacarandá, 8º andar, sala 873. Bairro Tamboré. Barueri, São Paulo. CEP: 06460-040, Brazil, Tel:
Abstract
Background: The study aimed to describe the results of Linda Lifetech’s thermography and mammography in the identification of breast cancer in an early detection scenario in Brazil. Methods: The data was cross-sectionally collected in 2021, in campaigns for the early detection of breast cancer in Brazilian municipalities. Women who underwent a mammogram participated in the study, and thermographic images were acquired by using Linda Lifetech. Results: 306 patients were screened. The mean age was 53.13 years old (SD 8.78), with the maximum born in the Southeast of Brazil (n= 125; 54.35%) and living in the state of São Paulo. 59.86% (95% CI 54.1 - 65.61) of women were in the menopause period and 17.65% (95% CI 13.38 - 21.92) had never undergone a mammogram before. The results for the mammogram for breast cancer showed 89.87%; 95% CI 86.49 - 93.25. The results of the mammogram showed that 84.97% had BI-RAD 2, 11.44% BI-RAD 1 and 0.98% BI-RAD 5. As the study's primary outcome, the proportion of suspect cases of breast cancer by mammography was 11 (3.59%) and non-suspect 295 (96.41%) cases. Suspect cases of Linda Lifetech’s thermography, according to mammography, were detected for the BI-RADS 4 (50%; 6 results) which predicts 95% of malignancy. Conclusion: Besides being a faster, painless method, without physical contact, without radiation emission, low cost and portable, Linda Lifetech’s thermography has similar trends as mammography in suspect and non-suspect cases. The similarity found can arise interest from health professionals, stakeholders, and patients, by being accessible, affordable, and less invasive, than other methods already in use. The study validates that the approach is equally accurate.
Keywords: Breast neoplasms, early detection of cancer, thermography, artificial intelligence
Introduction
Breast cancer is a public health concern worldwide [1]. It has become the most common diagnosed cancer globally and the most significant mortality factor among women [1-3]. In Brazil, it is most prevalent in women, being the leading cause of cancer death in the female population [4-5]. Improvements in breast cancer survival began to appear in the 80s, specifically in countries that adopted methods for early detection [1,6], [7]. The methods include early diagnosing people with signs and/or symptoms of the disease; and screening by applying a test or examination in a population without signs and symptoms suggestive of breast cancer, to identify changes suggestive of cancer [8].
Early detection has been proven to significantly reduce breast cancer mortality and have been widely incentivized due to its high potential for cure [1,6,7]. Mammography is the most used screening method globally, being considered the gold standard among imaging modalities for the screening of breast lesions [8,9]. It has contributed to the decrease breast cancer mortality worldwide, despite numerous disadvantages - including expensive equipments, radiation exposure, interval cancer (mainly due to breast density pattern), and overdiagnosis [8,10].
An alternative method for early detection of breast lesions is the thermography technology, which is a non-invasive and radiation-free imaging modality that measures the infrared radiation emitted by the body [11,12]. The principle of this method is to detect lesions with increased blood flow that, due to angiogenesis, are warmer than adjacent tissues, being expected to emit more infrared radiation than normal tissues [12].
Early attempts to use thermographic imaging of the breast as a tool in the detection of breast cancer date back to the 1970s [12]. This particular tool is outdated due to the lack of sensitivity and specificity of the cameras [12]. Today, high resolution camera produces excellent image and capture temperature variations of 0.02°C, reviving the interest in this method [12]. Among the advantages of this modality, thermographic images are easier to interpret than other breast imaging modalities, are cheaper and portable when compared to any other method (magnetic resonance imaging, ultrasound or mammography), and can improve early detection in less developed regions of the globe where current traditional methods are scarce [11-13].
Thus, Linda’s thermography explores novel route to detect cancer. It integrates the thermographic image of the breasts with an artificial intelligence (AI) algorithm for objectively interpreting the images and sense the cancerous tissue against healthy. It comprises of a software, associated with a thermal camera attached to a mobile phone, for AI platform communication. AI combined with thermal images seems to be an effective tool to detect breast cancer in early stage and has been providing significant predictability levels. Even so, recent literature shows numerous studies with divergent conclusions regarding thermography for breast cancer screening. Thus, the objective of this study is to describe the results of the Linda Lifetech’s thermography and mammography in the identification of breast cancer in an early detection scenario in municipalities of the States of São Paulo, Ceará and Paraíba, Brazil. The results may bring clarity on Linda Lifetech’s thermography potential and will provide guidance for future studies assessing accuracy and effectiveness of the device.
Materials and Methods
The study was carried out following the principles of the Helsinki Declaration and approved by (add certification number if you have). The importance of the study was explained to all participants and written consent was obtained from everyone. This was an observational cross-sectional study, and the data collected from February to November 2021 during campaigns for early detection of breast cancer in municipalities of the states of São Paulo, Paraíba and Ceará, Brazil. 322 women who underwent a mammogram were invited to take part in this study, and thermographic images were acquired by the Linda Lifetech device with AI-integrated software. The case history of individual patients were recorded by trained researchers using Google forms.
Eligibility criteria
To participate in the study, patients agreed and signed the Informed Consent Form (ICF). Women were included if aged ≥ 40 years old; underwent a mammogram for the early detection of breast cancer in municipalities in the states of São Paulo, Paraíba and Ceará during 2021; and who complied with the Linda Lifetech’s thermographic image acquisition protocol. The acquisition protocol of thermographic image consisted of requirements to ensure that the image is properly analyzed by the artificial intelligence model developed. It included an (1) ambient temperature between 21 and 23°C; (2) thermal harmonization of the patient, waiting 15 minutes in an air-conditioned environment; (3) the patient took off all clothing that covers the breasts (including the bra); (4) The patient with her hair tied back; (5) without any accessories on the neck (for example: necklaces, necklaces, etc.); (6) positioned against the wall; (7) The operator (who performed the image acquisition) positioned the device so as to frame the patient's breasts according to the grid displayed on the screen; (8) the patient should have her arms raised, interlacing her fingers behind her head. Exclusion criteria consisted of women breastfeeding, with flu and/or feverish state, in menstrual period; and with any inflammatory condition of the breasts.
The technology
Linda Lifetech consists of a thermography exam performed by a dedicated thermographic camera analyzed through an AI platform. Upon receiving the acquired image, the AI model divides the image vertically in half, starting to work with image A and image B. Next, the horizontal “flip” technique is applied to Image A so that it is compared with image B analyzing the colour pattern to identify similarities in their distribution. If a pattern disparity is detected, it is considered an indication of an abnormality. The same process is applied to image B. Subsequently, image A is submitted to the process of identification of colors per pixel, assigned a scale from 0 to 1 that considers the thermal body variation being from 25 to 36 degrees, where 0 is black/blue representing low-radiant areas and 1 is red/white representing hyperradiant areas. This decimal scale represents each image pixel as a thermal factor; for example, if a pixel is red/white, it can be between 0.9 and 1. In this way, areas with higher concentrations of hyperradiation can be identified compared to the same area of image B. The same process is applied to image B. Thus, thermal asymmetries are identified, which may indicate greater metabolic activity at certain points and, therefore, a suspicious location. Following the process, the results were provided in a dichotomous way (suspect or non-suspect), using a convolutional neural network (CNN) (Figure 1).
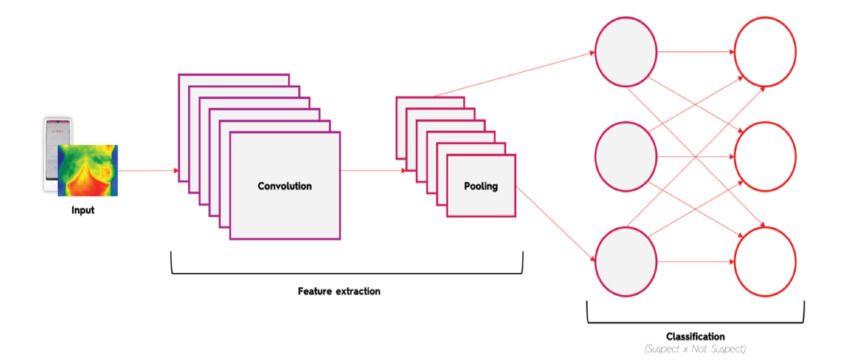
Figure 1.A simplified scheme of a convolutional neural network used to determine breast status (suspect or non- suspect).
The AI model
To assess the results of Linda’s thermography, a convolutional neural network (CNN) was used to determine breast status. In total, 1,898 images were collected during the campaigns, from the “Américas Amigas”, “Mulheres de Peito” and “CRIO” units. From the 1,898, 21 were discarded due to lack of integrity of the image. The 1,877 remaining images formed the study dataset. In this, 1,731 images were classified as non-suspect and 146 as suspect, according to the labels determined by the mammography results.
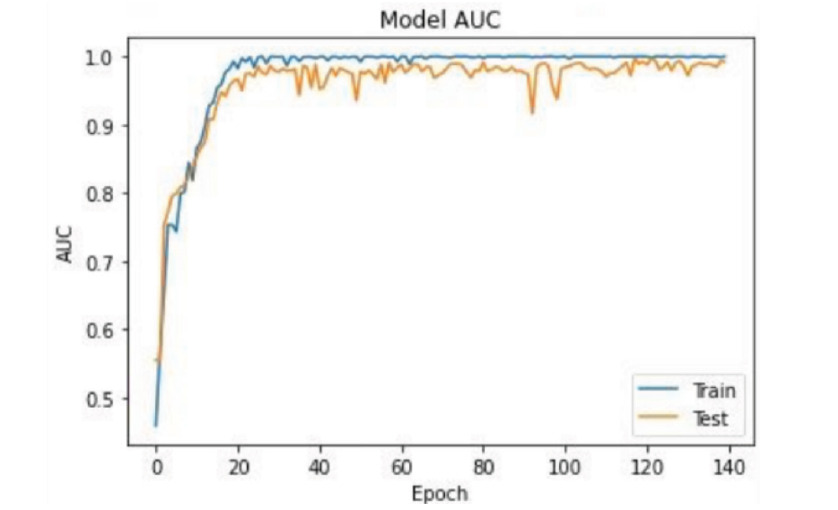
Figure 2.Area Under the Curve (AUC) of the model
The deep learning model was trained using the dataset of 1877 images (RGB with 240 x 320 pixels), which were split by a random algorithm into a training set (for the training of the model) and a testing group (for its validation), in proportions of 80% and 20%, respectively. The proportion of each group was not an arbitrary decision; it is commonly used proportion to train deep learning models [14]. The groups created were then used in all training runs so that every new model developed could be compared with the same training run.
In order to make the model more robust, we applied a random augmenter algorithm [15]. This was done with an algorithm that slightly distorted the original suspect images to create new ones. This technique allowed us to create 436 images, what increased our set of suspect images by random rotations of up to 10 degrees (up and bellow), random translations of the center and random expansions and contractions, of up to 10% of original dimensions [15-17]. The dataset augmentation led to a sample of 2313 images, segregated in 1849 images of training set and 464 images of testing set.
All the dataset was used to train the current production model; They were organized in batches of 128 images each (both training and validation). So, in the training set, there were 15 batches of 128 images and, in the validation set, 4 batches of 128 images. Both sets fed the model, but the validation set was used only to verify the performance of the training, at the end of each epoch. The training occurred in steps called epochs. Inside each epoch all the batches of 128 images were used to train the model. At the end of each epoch, the performance of the model was verified against the validation set. After many combinations of hyperparameters, for the training trials, the best values obtained were: batches with 128 images, 140 epochs, optimiser = RMSpro, learning rate of 0.001 and loss type = binary Crossentropy.
The metric used for training the model was the Area Under the ROC Curve, a metric commonly used for binary classification, which is our case. In this model, we obtained the AUC shown in Figure 2.
Descriptive analysis
For the descriptive analysis, only data from the testing set was used. The primary outcome of the descriptive study was the proportion of suspect cases of breast cancer by Linda Lifetech’s thermography and by mammography overall and according to clinical characteristics. Additionally, the proportion of suspect cases by Linda Lifetech’s thermography according to mammography BI- RAD category, age group and to the purpose of mammography performed (diagnosis or screening) were assessed as secondary outcomes. Variables as age, region of birth, city of residence, state where performed the exams, menopause, previous mammography, history of cancer in family (only first-degree relatives), previous breast surgery, reason for performing a mammography, mammogram result (B1 to B5) and thermogram result were also evaluated descriptively. Categorical variables were described by simple and crossed contingency tabulation, with frequencies and absolute percentages, and 95% confidence interval (CI). Continuous variables (quantitative variables) were summarized by the mean, standard deviation (SD), minimum, maximum, median and interquartile range (IQR) and 95% CI. Statistical analysis was conducted using Python version 3.6.9.
Results
Patient’s characteristics
In total, 322 eligible patients from the test et were screened, and 306 patients included in the descriptive analysis (Figure 3).
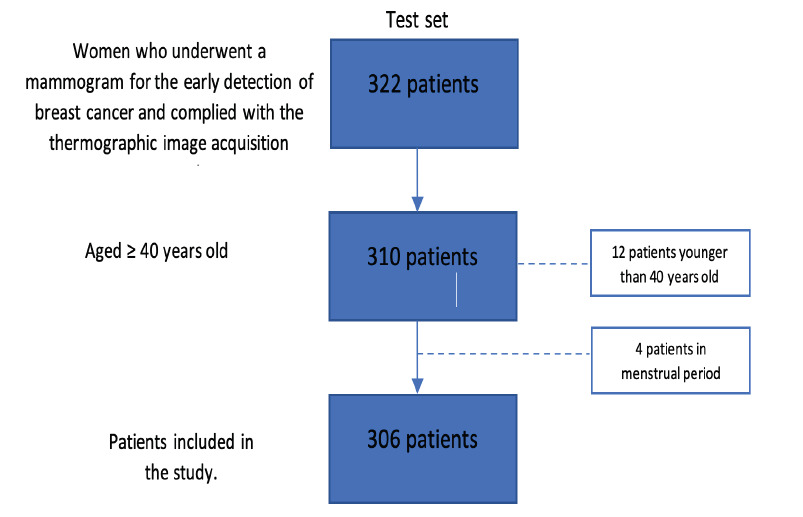
Figure 3.Patient’s eligibility1 1 Attrition related to the final testing set
The majority of patients were in the state of São Paulo (86.9%), followed by Ceará (14.7%) and Paraíba (3.6%). The mean of age of the patients was 53.13 years old (SD 8.78) and most of them were born in the Southeast of Brazil (54.35%) (Table 1). About the place of residence, most of them were living in the state of São Paulo, being São Paulo capital (101 participants) and Guarulhos (68 participants), those with about 55% of the participants of the study. In Ceará, all participants were from Fortaleza (42 participants). Paraíba had three participants, each of them from a different city (Table 1).
Table 1.Demographic characteristics of the participants
| Value | |
|---|---|
| n = 306 | |
| Age (years) | |
| Mean (SD) | 53.13 (8.78) |
| Median (IQR) | 52 (46 – 59) |
| Min-Max | 40 - 84 |
| Missing | 0 |
| Region of birth, N (%) | |
| North | 1 (0.43%) |
| Northeast | 98 (42.61%) |
| Midwest | 0 (0%) |
| South | 6 (2.61%) |
| Southeast | 125 (54.35%) |
| Missing | 0 |
| City of residence | |
| Ceará | |
| Fortaleza | 42 (13.73%) |
| Paraíba | |
| Barra De Santa Rosa | 1 (0.33%) |
| Campina Grande | 1 (0.33%) |
| Pedra Lavadra | 1 (0.33%) |
| São Paulo | |
| Ferraz De Vasconcelos | 11 (3.59%) |
| Guarulhos | 68 (22.22%) |
| Itaquaquecetuba | 13 (4.25%) |
| Maua | 59 (19.28%) |
| Praia Grande | 1 (0.33%) |
| Ribeirao Pires | 6 (1.96%) |
| Santo Andre | 1 (0.33%) |
| Sao Paulo | 101 (33.01%) |
| Suzano | 1 (0.33%) |
SD: Standard deviation, IQR: interquartile range
Regarding clinical characteristics, 59.86% (95% CI 54.1 - 65.61) were during menopause period and, for those who were not during menopause, the mean time since the last period was 42.34 (SD 48.77) days (Table 2). Regarding previous mammograms, 17.65% (95% CI 13.38 - 21.92) have never done a mammogram before and for those who have previously done, 47.39% (95% CI 41.79 - 52.98) did it more than two years ago and had a normal result; Only 0.65% (95% CI 0.0 - 1.56) had it done two years ago and with a changed result. The remaining performed it less than two years ago, being 3.27% (95% CI 1.28 - 5.26) with changed results and 31.05% (95% CI 25.86 - 36.23) with normal results. The major purpose to perform the mammogram was screening (89.87%; 95% CI 86.49 - 93.25) (Table 2). For risk of cancer in the family, 25.66% (95% CI 20.75 - 30.57) had the history of having a family member with cancer in the past. The great majority of the participants included have never done a breast surgery (96.08%; 95% CI 93.9 - 98.25); Among those who have, most part did it for aesthetics or due to a benign tumor (Table 2).
Table 2.Clinical characteristics of the participants
| n = 306 | ||
|---|---|---|
| Menopause, N (%) Yes | ||
| 167 (59.86%) | [ 54.1 - 65.61] | |
| No | 112 (40.14%) | [ 34.39 - 45.9] |
| Missing | 27 | - |
| Menopause age | ||
| Mean | 17.42 | - |
| SD | 16.29 | - |
| Median | 10 | - |
| IQR | Apr-25 | - |
| Min-Max | Jan-58 | - |
| Missing | 145 | - |
| Time since last period (days) Mean | ||
| 42.34 | - | |
| SD | 48.77 | - |
| Median | 31 | - |
| IQR | 23 - 40 | - |
| Min-Max | 11 - 296 | - |
| Missing | 189 | - |
| Previous mammogram, N (%) Never did | 54 (17.65%) | [ 13.38 - 21.92] |
| Yes | ||
| > 2 years ago - changed result | 2 (0.65%) | [ 0.0 - 1.56] |
| > 2 years ago - normal result | 145 (47.39%) | [ 41.79 - 52.98] |
| < 2 years ago - changed result | 10 (3.27%) | [ 1.28 - 5.26] |
| < 2 years ago - normal result | 95 (31.05%) | [ 25.86 - 36.23] |
| Missing | 0 | |
| Risk of cancer in family, N (%) No | 226 (74.34%) | [ 69.43 - 79.25] |
| Yes | 78 (25.66%) | [ 20.75 - 30.57] |
| Missing | 1 | - |
| History of breast surgery, N (%) No | 294 (96.08%) | [ 93.9 - 98.25] |
| Yes | ||
| Benign/both breasts | 1 (0.33%) | [ 0.0 - 0.97] |
| Benign/right breast | 5 (1.63%) | [ 0.21 - 3.05] |
| Benign/left breast | 2 (0.65%) | - |
| Breast cancer/right breast | 1 (0.33%) | [ 0.0 - 0.97] |
| Breast cancer/left breast | 0 (0%) | - |
| Aesthetics | 3 (0.98%) | [ 0.0 - 2.08] |
| Missing | 0 | |
| Type of mammogram performed, N (%) Diagnosis | 31 (10.13%) | [ 6.75 - 13.51] |
| Screening | 275 (89.87%) | [ 86.49 - 93.25] |
| Mammogram result, N (%) BI-RAD 0 | 0 (0%) | |
| BI-RAD 1 | 35 (11.44%) | [ 7.87 - 15.0] |
| BI-RAD 2 | 260 (84.97%) | [ 80.96 - 88.97] |
| BI-RAD 3 | 0 (0%) | - |
| BI-RAD 4 | 8 (2,61%) | [ 0.83 - 4.4] |
| BI-RAD 5 | 3 (0.98%) | [ 0.0 - 2.08] |
| Without report/Missing | 0 | - |
Regarding the results of Linda Lifetech’s thermography 12 (3.92%) were suspect and 294 (96.08%) non-suspect cases, while the proportion of suspect cases by mammography was 11 (3.59%) and non-suspect 295 (96.41%) cases (Table 3).
Table 3.Proportion of suspect cases of breast cancer by Linda Lifetech’s thermography and by mammography
| Suspect | Non-suspect | Total available information | |
|---|---|---|---|
| N (%) | N (%) | N (%) | |
| Linda Lifetech’s | |||
| thermography | 12 (3.92%) | 294 (96.08%) | 306 (100%) |
| Mammography | 11 (3.59%) | 295 (96.41%) | 306 (100%) |
Although the proportion of suspect and non-suspect cases were similar among the both methods, when Linda Lifetech’s thermography results were grouped according to mammography results (considering the BI-RAD classification: non-suspect as BI-RAD 1, 2 and 3; suspect as BI-RAD 4 and 5), 35 cases (11.4%) of BI-RAD 1 and 257 cases (83.99%) were classified as non-suspect by Linda Lifetech’s thermography and 6 cases 1.96%) of BI-RAD 4 and 3 cases (0.98%) of BI-RAD 5 as suspect. However, three cases (0.98%) of BI-RAD 2 were classified by Linda Lifetech’s thermography as suspect and two cases (0.65%) of BI-RAD 4 as non-suspect (Table 4).
Table 4.Linda Lifetech’s thermography results grouped by mammography results
| Linda Lifetech’s thermography | Mammography | N (%) |
|---|---|---|
| Non-suspect | BI-RADS 1 | 35 (11.44%) |
| Non-suspect | BI-RADS 2 | 257 |
| (83.99%) | ||
| Non-suspect | BI-RADS 4 | 2 (0.65%) |
| Suspect | BI-RADS 2 | 3 (0.98%) |
| Suspect | BI-RADS 4 | 6 (1.96%) |
| Suspect | BI-RADS 5 | 3 (0.98%) |
According to the mammography results (BI-RADS 1, 2, 3, 4 and 5), 12 cases were considered suspect and 272 non-suspects. Suspect results of Linda Lifetech’s thermography according to mammography were mostly concentrated on BI-RADS 4 (50%; 6 results), followed by BI-RADS 3 and 5 (25% each, 3 results). On other hand, for non-suspect results in Linda Lifetech’s thermography, the majority was defined as BI-RAD 2 (87.41%; 257 results) (Table 5).
Table 5.Proportion of suspect cases by Linda Lifetech’s thermography according to BI-RAD, age group and purpose of the thermography
| Linda Lifetech’s thermography result | ||
|---|---|---|
| Suspect | Non suspect | |
| N = 12 | N = 272 | |
| According to mammography result, N (%) | ||
| BI-RAD 1 | 0 (0%) | 35 (11,9%) |
| BI-RAD 2 | 3 (25%) | 257 (87,41%) |
| BI-RAD 3 | 0 (0%) | 0 (0%) |
| BI-RAD 4 | 6 (50%) | 2 (0,68%) |
| BI-RAD 5 | 3 (25%) | 0 (0%) |
| Missing | 0 | 0 |
| According to age group, N (%) | ||
| 40-49 y.o. | 2 (16.67%) | 120 (40,82%) |
| 50-59 y.o. | 6 (50%) | 107 (36,39%) |
| 60-69 y.o. | 4 (33.33%) | 51 (17,35%) |
| 70-85 y.o. | 0 (0%) | 0 (0%) |
| According to the purpose of the mammography, N (%) | ||
| Screening | 3 (25%) | 272 (92,52%) |
| Diagnosis | 9 (75%) | 22 (7,48%) |
| Missing | 0 | 0 |
Suspect cases in Linda Lifetech’s thermography were more common from 50 to 59 years of old (50%), followed by the age group 60 to 69 years old (33.33%). Non-suspect cases, however, were more frequent among the age group of 40 to 49 years old (40.82%) (Table 5). Regarding the purpose of the exam (screening or diagnosis), 75% of the suspect cases had diagnostic purposes, and among non-suspects, 92.52% had screening purpose (Table 5).
Discussion
In our study, women aged 40 years old or more who have registered to perform a mammogram were invited to perform Linda Lifetech’s thermography. It was carried out during campaigns for early detection of breast cancer in the states of São Paulo, Paraíba and Pernambuco. As a result, we found that both methods (mammography and Linda Lifetech’s thermography) had a similar trend regarding the behavior of suspected cases, even considering clinical characteristics distribution. Few suspect results by mammography were not found as suspect by Linda Lifetech’s thermography.
Breast cancer is the most incident cancer in women worldwide [18]. It significantly affects female’s morbidity and mortality, that tend to grow progressively from the age of 40 onwards [10, 18]. One effective strategy to prevent the disease spread and improve the survival rates is the early detection [1,6,10]. Breast cancer diagnosed earlier is usually associated to better disease prognosis, more effective treatments and less associated disease [19]. This makes the screening of breast cancer one of the most important public policies in the world, being recommended by the World Health Organization (WHO) [20]. Linda Lifetech’s thermography have the potential to boost public policies that aim in increasing the chances of earlier breast cancer detection in Brazil.
Although other methods were developed for breast cancer screening, mammography remains the gold standard since the 1960s. It can detect the disease in early stage, resulting in a reduced mortality by 25% [10]. However, it is still a method that faces many challenges when it comes to wide spread implementation. For example, policies for screening states that it should be offered to women from 50 to 69 years old, only once every two years to mitigate risks [19]. In addition, it is a large and expensive equipment, making resource allocation in healthcare services an incredible challenge [21,22]. In Brazil, not only imaging clinic or hospitals are not available in all municipalities, but, in July 2022, only 1,335 municipalities from the 5,570 in the country had at least one mammogram, requiring patients to move to the nearest reference center [23,24]. Considering the Brazilian scenario itself, regional disparities in the accessibility of health care is a reality, with most of the healthcare resources being concentrated in the Southeast and Northeast [25].
In the context of screening, alternatives are imperative to reduce disparities in mammography access, especially in continental countries, like Brazil. Linda Lifetech uses thermography, which is a method that can help to improve the screening of breast cancer, and can be an auxiliar method to the disadvantages of mammogram [26]. It might be useful in increasing the availability and coverage of screening strategies, since accessibility of thermography might be an advantage for screening in rural areas and places in which mammography and other techniques are not available. In addition, it can be an option to opportunistic screening, a strategy of early detection shown to be effective in the absence of organized screening. A study has documented that the higher the prevalence of opportunistic screening, the more favorable breast cancer prognosis are observed in the population [27]. In the logic of the health system organization, an affordable and accessible alternative may provide a positive impact for health system, since it would avoid exacerbated referrals, being cheaper and simple to manage. Also, it is a non-invasive and painless method that does not involve compression of the breast or exposure to radiation, being an alternative for younger women at risk, in which mammography is not recommended due to the breast density, that makes the detection of cancer by mammography difficult [12, 19, 28, 29].
Despite the criticism and loss of interest in thermography’s accuracy in the past, advances and maturation of thermography industry have been allowing excellent images to be obtained, with high resolution cameras, increasing sensitivity for detecting 0.02 C variations in temperature [12].
It should be noted that even mammography is not a 100% accurate exam, and it can be lower among women with higher breast density. Also, almost 40% of women included had 40 to 49 years old, younger than the age group who is usually offered screening (50–69 years) [20]. Finally, we should consider that biopsy is the most accurate method to identify real cases, but it is an invasive procedure that should be performed only when there is a suspicious finding [30, 31].
Our study has limitations that should be mentioned. Due to data availability, this study did not allow to assess the accuracy and performance of Linda Lifetech’s device. This is a descriptive study assessing participants characteristics and their findings of thermographic image and mammography performed in the population who performed mammograms in selected sites during 2021. Considering that a convenience sample was used, the results do not have external validity – meaning that they might not reflect the result found in a similar study when conducted in a different population. Even considering that we used a convenience sample and that it has limitations in extrapolating the results to other populations, this study was performed in campaigns for early detection to identify the potential of Linda Lifetech’s thermography in front of mammography – which justifies the way the sample was chosen and its size. Also, that might not be a balance between the training and test settings used, as well as selection bias that did not allow to assess the method's accuracy.
The strength of the presented study is a real-world scenario with women with broad demographics and clinical characteristics - it can provide insights for future investigations to improve early and opportune breast cancer diagnosis.
Conclusion
Linda Lifetech’s thermography and mammography had similar trends of suspect and non-suspect cases. As much as the study does not bring inference results, based on non-inferiority results of Linda Lifetech’s thermography in the face of mammography, the similar trend of suspect cases among both methods will be useful to raise hypotheses to perform future studies. In addition, the similarity found can raise interest from health professionals, stakeholders, and patients, by being accessible, affordable, less invasive, and painful than other methods already in use.
Abbreviations
AI: artificial intelligence; ICF: Informed Consent Form; CNN: convolutional neural network; CI: confidence interval; SD: standard deviation; IQR: interquartile range; WHO: World Health Organization
Acknowledgments
IQVIA Brazil provided statistical and writing assistance for the manuscript. Termo Health Tecnologia Ltda funded this study.
Ethics
The study was approved by a Brazilian ethics committee. Ethical approval was issued by the Research Ethics Committee of Hospital Moriah, under the Certificate of Presentation of Ethical Appreciation CAE 29969120.0.0000.8054, numbers 4.809.786 and 4.617.348.
Conflicts of Interest
Rubens Fernando de Lima Mendrone and Daniella Bucci are, respectively, Founder and Technical Responsible for LINDA Lifetech.
References
1. World Health Organization, “Breast cancer,” WHO Fact Sheets. https://www.who.int/news- room/fact-sheets/detail/breast-cancer (accessed May 11, 2022).
2. World Health Organization. “Breast cancer now most common form of cancer: WHO taking action.” https://www.who.int/news/item/03-02-2021-breast-cancer-now-most-common- form-of-cancer-who-taking-action (accessed May 11, 2022).
3. E Warner. “Breast-Cancer Screening”. N Engl J Med. 2011; 365:1025–1032. doi: 10.1056/NEJMcp1101540.
4. Instituto Nacional De Câncer José Alencar Gomes Da Silva. “Estimativa 2020: incidência do Câncer no Brasil.” 2019. https://www.inca.gov.br/estimativa/taxas- ajustadas/neoplasia-maligna-da-mama-feminina-e-colo-do-utero (accessed May 12, 2021).
5. Instituto Nacional De Câncer José Alencar Gomes Da Silva. “Atlas da mortalidade.,” Database, 2022. https://www.inca.gov.br/app/mortalidade (accessed Jul. 21, 2022).
6. L Wang. “Early Diagnosis of Breast Cancer”. 2017, doi: 10.3390/s17071572.
7. E Detection. “Knowledge into Action Early Detection Cancer Control Knowledge into Action Early Detection.”
8. Brasil. Ministério da Saúde. Instituto Nacional de Câncer (INCA), “PARÂMETROS TÉCNICOS PARA O RASTREAMENTO DO CÂNCER DE MAMA,” Rio de Janeiro, 2021.
9. P Zubor. “Why the Gold Standard Approach by Mammography Demands Extension by Multiomics ? Application of Liquid Biopsy miRNA Profiles to Breast Cancer Disease Management,” 2019.
10. M Abdulla, S Al. “A Systematic Review of Breast Cancer Detection Using Thermography and Neural Networks.” 8: 2020, doi: 10.1109/ACCESS.2020.3038817.
11. M Rassiwala, P Mathur, R Mathur, et al. “Evaluation of digital infra e red thermal imaging as an adjunctive screening method for breast carcinoma : A pilot study.” Int J Surg. 2014; 12: 1439–1443. doi: 10.1016/j.ijsu.2014.10.010.
12. DA Kennedy, T Lee, D Seely. “A Comparative Review of Thermography as a Breast Cancer Screening Technique.” 2009; 9–16.
13. AI Majeed, N Naz, M Arif, et al. “Diagnostic Accuracy of Mammography in Detecting Breast Cancer Keeping Histopathology as Gold Standard.” 2016; 118–121.
14. HH Rashidi, NK Tran, EV Betts, et al. “Artificial Intelligence and Machine Learning in Pathology: The Present Landscape of Supervised Methods.” Acad Pathol. 2019; 6: 2374289519873088. doi: 10.1177/2374289519873088.
15. P Chlap, H Min, N Vandenberg, et al. “A review of medical image data augmentation techniques for deep learning applications.” J Med Imaging Radia Oncol. 2021; 65: 545–563. doi: 10.1111/1754- 9485.13261.
16. T Devries, G W Taylor. “Dataset Augmentation in Feature Space.” 2017; 1–12. [Online]. Available: https://arxiv.org/abs/1702.05538.
17. C Shorten, TM Khoshgoftaar. “A survey on Image Data Augmentation for Deep Learning.” J Big Data. 2019. doi: 10.1186/s40537-019-0197-0.
18. Brasil. Ministério da Saúde. Instituto Nacional de Câncer (INCA), “Estimativa 2020: incidência do Câncer no Brasil,” 2019. https://www.inca.gov.br/sites/ufu.sti.inca.local/files//media/document//estimativa-2020- incidencia-de-cancer-no-brasil.pdf.
19. Brasil. Ministério da Saúde. Instituto Nacional de Câncer (INCA), “Detecção precoce.” https://www.inca.gov.br/controle-do-cancer-de-mama/acoes-de-controle/deteccao- precoce.
20. I Handbooks, C Prevention, IARC HANDBOOKS Breast Cancer Screening IARC HANDBOOKS Breast Cancer Screening. 15.
21. E Caetano. Inequalities in socioeconomic status and race and the odds of undergoing a mammogram in Brazil. Int J Equity Health. 2016; 1–16. doi: 10.1186/s12939-016-0435-4.
22. N Alves. “Treatment delays among women with breast cancer in a low socio- economic status region in Brazil.”2017; 4–11. doi: 10.1186/s12905-016-0359-6.
23. Brasil. Ministério da Saúde. Instituto Nacional de Cancer (INCA), Sistemas de Informação do Controle do Câncer de Mama (Sismama) e do Câncer do Colo do Útero (Siscolo). Manual Gerencial. Rio de Janeiro, 2011.
24. Brasil. Ministério da saúde. DATASUS, “Cadastro Nacional de Estabelecimentos de Saúde.” http://cnes.saude.gov.br/.
25. B D P Fonseca, PC Albuquerque, DF Saldanha, et al. “Articles Geographic accessibility to cancer treatment in Brazil : A network analysis.” Lancet Reg Heal Am. 2022; 7: 100153. doi: 10.1016/j.lana.2021.100153.
26. JL Gonzalez-Hernandez, AN Recinella, SG Kandlikar, et al. “Technology, application and potential of dynamic breast thermography for the detection of breast cancer.” Int. J. Heat Mass Transf. 2019; 131: 558–573. doi: https://doi.org/10.1016/j.ijheatmasstransfer.2018.11.089.
27. J Bulliard, C Ducros, C Jemelin, et al. “Effectiveness of organised versus opportunistic mammography screening.” Ann Oncol. 2009; 20: 1199–1202. doi: 10.1093/annonc/mdn770.
28. L Sun, J Stone, E Fishell. “Mammographic Density and the Risk and Detection of Breast Cancer.”2007; 227–236.
29. PR Pavithra, KS Ravichandran, KR Sekar, et al. “The effect of thermography on breast cancer detection.” Syst Rev Pharm. 2018; 9: 10–16. doi: 10.5530/srp.2018.1.3.
30. American Cancer Society, “Breast Biopsy.” https://www.cancer.org/cancer/breast- cancer/screening-tests-and-early-detection/breast-biopsy.html#:~:text=A core needle biopsy (CNB, if breast cancer is suspected.
31. MF Dillon, CM Quinn, EW McDermott, et al. “Diagnostic accuracy of core biopsy for ductal carcinoma in situ and its implications for surgical practice.” 2006; 740–743. doi: 10.1136/jcp.2005.034330.
Received: January 30, 2022;
Accepted: February 20, 2023;
Published: February 22, 2023.
To cite this article : de Lima Mendrone RF, Mazoni AF, do Espírito Santo JMR, et al. Application of Thermography and Neural Network Classification for Early Detection of Breast Cancer. British Journal of Cancer Research. 2023; 6(1): 597- 603. doi: 10.31488/bjcr.181.
©2023 de Lima Mendrone RF, et al.
