Research article / Open Access
DOI: 10.31488/bjcr.185
Arabic Modified Version of Palliative Outcome Scale (POS) Cross Cultural Adaptation and Validation
Asma Mohammad Al Bulushi*1,2, Jessie Johnson4, Zeinab Idris1,2, Azza Adel Ibrahim Hassan1,3, Nima Ali1, Shaikhah Mohsen Al keldi1, Khadra Sofi Yassin1, Ayman Allam1
1. National Center for Cancer Care and Research, Hamad Medical Corporation, Doha, Qatar
2. University of Calgary-Qatar, Doha, Qatar
3. Cancer Management & Research, Medical Research Institute, Alexandria University, Alexandria, Egypt
4. Beal University, Canada
*Corresponding author:Asma Mohammad Al Bulushi, National Center for Cancer Care and Research, Hamad Medical Corporation, Doha, Qatar, Tel:00974 55441828
Abstract
Palliative Outcome Scale (POS) is an important tool to assess needs and priority of care in palliative care settings, it was developed to assess cancer patients; quality of life (QOL). The aim of this study is translation of the POS to Arabic using the seven phases steps as outlined by the palliative care outcome scale (POS) family of measures manual for translation, carry out a cross-cultural adaptation of the POS for Arabic-speaking patients.After the translation phases, a focus group interview was conducted with seven health care providers and interview was performed on five patients and five caregivers. Then the result of the focus group and interview were integrated, and final format of tool was developed.We conducted 10face to face cognitive interviews with patients and caregivers, and one focus groups with seven health care providers. As a result, patient, caregivers, and health care providers accepted the feasibility of POS Arabic version translation, but some minor feedbacks on rewording and meaning of the sentences were raised for adaption. The team were successful in achieving the goal of translating and culturally adapting an Arabic-version POS tool.The POS Arabic version scale is a valid and reliable outcome measure. It can assess, monitor symptoms, QOL needs and concerns in advance cancer patients in palliative setting. We recommend future research on the Arabic version of the POS tool with a focus on psychometric analysis and clinical implication in a larger group of our Arabic speaking palliative care patients.
Key words: Quality of life, end of life, palliative care, symptoms burden
Introduction
The quality of life (QOL) is a centre of focus in palliative care. Because of the distinctive cultural norms and values of the people who live in Middle East region, the information on QOL has relatively special in its features [1].Advanced cancer patients suffer from a burden of symptoms at the end of life, which need proper assessment by a reliable tool.
Cancer patients with advance disease have increased burden of symptoms at the end of life. The proper assessment and evaluation of advanced cancer patient symptoms is considered a gold standard for proper management and to improve quality of life of cancer patients at end of life. There are different aspects of QOL that need to be assessed such as physical including symptoms burden, emotional, psychological, and spiritual [2]. Assessing the psychological and mental health for cancer patients; in addition to their physical symptoms is a crucial element for advanced cancer patients. Increased level of stress and anxiety have ab negative impact on cancer patient; QOL. Moreover, there is a need to assess the spiritual aspect of cancer patients to guide the provided care and meet the spiritual needs for cancer patients. Identification of these needs are best assessed through the POS tool.
In 1999 the Palliative Care Core Audit Project Advisory Group in the United Kingdomdeveloped Palliative Care Outcome Scale POS instrument to assess advanced cancerpatients’ QOL for palliative patients and it was developed as outcome measurement for patients’ symptoms [3].(See online Supplementary material). The POS is a self-administered tool which includes 10 multidimensional items. The POS scale is intendedto measure different aspects of QOL such as the physical, psychological, psychosocial,emotional, spiritual, practical, and different domains of cancer patients’ QOL [4,5].The integrated POS scale is used to assist in the identification of active and ongoing problems thatmay be faced by cancer patients throughout the disease journey [6].
The aim of this study is translation of the POS to Arabic using the seven phases steps as outlined by the palliative care outcome scale (POS) family of measures manual for translation, carry out a cross-cultural adaptation of the POS for Arabic-speaking patients.
Methods
The research team followed the guidelines included in thepalliative outcome scale (POS) manual set forth by the Antunes et al. [7].These guidelines were prescriptive and based on expert well established standard translation and validation protocols. The guidelines included in the manual were laid out in eight phases. These included the conceptual definition, forward and backward translation, translation, expert review, conceptual cognitive debriefing, proof reading, validation and audit, psychometric testing and finally report and publication(Figure 1) [7].
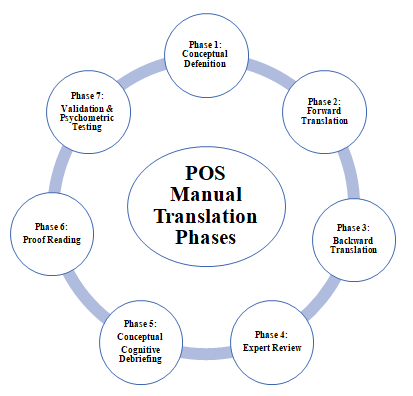
Figure 1:POS Manual Translation Phases
Translation
After obtaining consent to translate the POS to Arabic we enlisted the help of a translation company who had staff who spoke both English and Arabic. Two independent people who were not in the clinical setting translated the tool to Arabic. Once translated, the tool was sent to two independent Arabic speaking translators one clinician and one lay person who compared the original POS document for clarity. The translators met to discuss items on the scale and took notes on areas that were not clear. The translated scale was then sent to two other independent English speakers who back translated the scale to English. Once the two versions were compared, the scale was further translated back to Arabic. The Arabic version which had gone through forward, back and forward translation was then reviewed by both a palliative care physician, palliative care nurse and a person who worked in the palliative care area but was a non-clinician. The staff reviewing the Arabic translated version were asked various questions such as 1. Were the questions written the same as the ones intended, 2. Was there any incorrect wording that needed changing for ease of clarity. It was noted thatquestions were correct in their translation however an alternative word or phrase could have been used for clarity in some instances.
Setting and participants
The National Cancer Care Centre of Research (NCCCR) was the backdrop of this translation and testing of the POS. This centre consists of one ward where patients who are nearing the end of life reside. Currently the ward has 10 palliative care beds. The team that provides services is interprofessional consisting of nurses, physicians, social workers etc. Both staff, patients and caregivers were either interviewed or participated in focus groups to obtain validation of the scales. Patients on the palliative care ward consisted of anyone with a diagnosis of cancer and where death was imminent. Caregivers consisted of any immediate or extended family or friend that was the most responsible person for the patient. Patients and caregivers were interviewed one on one to obtain their perception of the understanding of the translated version of the scale (n=5) and caregivers (n=5). Staff included in the validation of the scale were direct care staff to the patients on the ward and who had been familiar with the palliative outcome scale. Staff validation was conducted using focus groups (n=7).
Cognitive testing
The next phase consisted of cognitive one to oneinterview with eligible patients (n=5) and caregivers (n=5) and a focus group of healthcare providers (n=7). The next phase consisted of proofreading the scale.For this research purpose verbal informed consent was used for cognitive testing phase focus group and participant’s interview in accordance to ethical guidelines and regulationsand approved by institutional review board in the Medical Research Centre in Qatar. The aim of this phase was to administer the tool to palliative patients and palliative staff to test the Arabic translated version of the tool and to measure inter-rater reliability and content validity. This is another way to ensure questionnaire clarity and to ensure that the items and scoring had been correctly translated.Inclusion criteria were patients with advanced cancer over the age of 18, able to give consent freely, diagnosed with a life limiting illness and read, write, and understand Arabic. Any patient who was near death, distressed and was unable to read, write or understand Arabic was excluded from the study. For the interviews with patients, purposive sampling was used. Once the scale had been administered to the patients and staff, discussion regarding the clarity and accuracy of the scale was completed to note any discrepancies within the scale. All points and any suggestions raised from the focus group and interviews were sent to the POS development team for proof reading and agreement.
Data analysis
Analysis was completed by two of the research members by immersing themselves within the data. This included reading and rereading the transcripts to become familiar with the data. Once these two research members were familiar with the data they began to focus on each item of the scale. All facets of the translated version of the scale were reviewed and discussed in terms of how they were worded and meaning. Once this was complete both researchers began to thematically analyse the information to come up with themes. This was done by reviewing information related to the participants perception of the questions.
Ethics approval was sought, and approval gained from Hamad Medical Corporation, certificate number MRC-01-17-172. All methods carried out in the study were performed in accordance with Medical Research Centre (MRC) ethical relevant guidelines and regulations.
Results
Of note, is that our sample size is relatively small andall participants either worked for or were caregivers and patients of the palliative care ward at NCCCR demographic data included is presented below tables. Patient information data such as age, gender and diagnosis presented in table 1.Table 2 presented information about staffs who participated in focus groups.
Table 1.Patient Information
| Diagnosis | Age range/Gender |
|---|---|
| Cervical Cancer | 53 Female |
| Hepatocellular Cancer | 57 Male |
| Bladder Cancer | 78 Male |
| Colon Cancer | 42 Female |
| Colon Cancer | 51 Male |
Table 2.Staff who participated in the study doing interviews and focus groups
| Profession | Profession | Role in the study |
|---|---|---|
| Nurse | Clinical Nurse Specialist Cardio Oncology Clinical | Interviewer/ focus group |
| Nurse | Cardio Oncology Clinical Palliative care unit Nurse Specialist | Focus group |
| Nurse | PCU | Focus group |
| Nurse | Staff Nurse Palliative Care unit NCCCR | Focus group |
| Physician | Associate Consultant NCCCR | Focus group |
| Physician | Associate Consultant NCCCR | Focus group |
| Physician | Consultant in PICU | Focus group |
During the one-to-one interview with the patient, it was noted they felt positively towards the tool and the fact it was translated for use in Arabic.Most felt the tool was user friendly and easily understood. Below is the scale and its interpretation from both patients and staff. Feedback from patient, carer and staffs comprehension of the pre-final POS questions and also questions being revised (Supplementary Table 1).
In the final phaseof proof reading: The developed version of the Arabic POS and the documents of each phase (such as the pre-final translations and final translation documents) was sent to the POS development team for the proof-read agreement and for psychometric testing and validation. The final corrected version was used afterwards for cognitive interviews.
Discussion
The POS tool was first developed and validated in English by Hearn J. in 1999 at King’s College School ofMedicineAnd dentistry and St. Christopher’s Hospice London. It is considered a comprehensive tool that addresses physical, psychological, and spiritual domains pertinent to palliative care in patients with advanced cancer diagnosis, or patients with irreversible terminal diseases of the heart, kidney, liver or neurological diseases [8].
Since then, the POS tool has been translated and validate it into 12 different languages. The primary aim of the present study was to translate POS into Arabic language. This aim has been successfully achieved by following the seven of the eight internationally approved phases of translation (conceptual definition, forward and backward translation, expert review, conceptual cognitive debriefing, proof reading, validation and psychometric testing, report, and publication). Of note, is that the sample size is too small to carry out psychometrics of the translated POS scale.
About 50-60% of our patients at the National Centre for Cancer Care and Research (NCCC R) are Arabic speaking, hence the importance of translating and validating the POS tool into Arabic language. It is worth noting that thisstudy,is the first study translating POS into Arabic language.In the present study, there was a good overall comprehension of the questions included in the POS tool among patients, careers, and the focus of group (12 questions). Question #4 family/ friends’anxiety/worry was reported to be confusing by the focus group in relation to “feelings and its relationship to mood” , as well as question #7 (life worth living) which was found a difficult question to ask the patients if he or she feels they deserve to live and suggestion was to rephrase such questions in Arabic to ask about patients’ acceptance or coping with their condition. Finally, question 9(time wasted on healthcare appointments )and question 10 (practical matters addressed); the focus group felt that the three days’ time frame in both questions might not be applicable and that adjustments are needed in both questions to address those issues.
Such difficulties in translation to another language have been similarly found in the literature. In 2019,Veronese et al.were able to translate and culturally adapt the integrated palliative outcome scale (IPOS)into the Italian language [9].They faced some layout problems during the process of translation mainly regarding the classification of the meaning of choices and some cultural interpretation of some questions and responseoptions and interpretation ofsome instructions. However, with the use of some new terms considered to be more appropriate and comprehensive to the Italian culture, they were able to reach face and content validity of the translated (IPOS) version for use in clinical settings among Italian speaking patients, making the tool also ready for the psychometric validation.
The same findings were reported later in 2022 by Martinsson and Sahlen in their publication concerning translation and cultural adaptation of IPOS to the Swedish language [10].In this study, they focused on people with dementia under palliative care. All the focus group (13 Staff) were nurses or assistant nurses.They faced some problems with questions pertaining to the Swedish translation of “skin tearing”, explanation of “wondering” also the Swedish explanation of “nausea and vomiting”, the Swedish term of “overwhelming” which was translated as “very serious” or “unbearable” in the backward translation.However, the study was able to reach its primary aim of translating and culturally adapt the IPOS to the Swedish language with some minor necessary modifications.
In 2022 Spichiger published thetranslated version of the IPOS tool, to the Swiss- German languages among patients with dementia in an easy language adaptation and translation version [11].They usedthe six-phase process in translation. They also faced minor challenges and backward translation of some of the original questions which could be solved by minor adjustments and rewording some phrases into an easier and more understandable language.
To the best of our knowledge, our present study is the first one to translate and culturally adapt the original POS tool into Arabic language, that will assist in the caregiving of a large percentage of our patients with cancer diagnosis. We elected to postpone psychometric analysis in our present study for future research which will include a larger number of patients, also aiming to prospectively report on the valueof applying the translated POS in clinical practice.
Conclusion
In conclusion, we were able to reach our primary aim of translating and culturally adapting an Arabic translated version of the Pos tool. This will serve a large percentage of our Arabic speaking palliative care patients. Our future research will focus on psychometric analysis and clinical implication of the translated POS tool in a larger group of our Arabic speaking palliative care patients. The POS Arabic version scale is a valid and reliable outcome measure, both in patient self-report, caregivers, staff proxy-report versions. It can assess, monitor symptoms, QOL needs and concerns in advance cancer patients in palliative setting.
Abbreviations
POS: Palliative outcome Scale; QOL: Quality of life; NCCCR: National Center of Cancer care and Research; IPOS: Integrated palliative outcome scale
Declaration
Ethics approval and consent to participate
The ethical approval for study and verbal informed consent has been obtained on 20, March 2020 from ethics committee of Medical Research Center(MRC)- Qatar after completion of the ethical guidelines and regulation of Hamad Medical Corporation with reference ID MRC-01-17-172. All methods carried out in the study were performed in accordance with MRC ethical relevant guidelines and regulations. For this research purpose the verbal informed consent was used for cognitive testing phase focus group and participant’s interview in accordance to ethical
guidelines and regulations and approved by institutional review board in the Medical Research Centre in
Qatar. It was obtained from the participants after explaining the study. Information sheet was given to all participants, who agree to join the study, and met the inclusion criteria, they were approached by one of the researches team. The research member explained about the study nature, purpose, risk, and benefits. Participants informed that their participation is voluntary. They were asked to review and feedback on scale by a research team member. Also, the participantswere informed that they can withdraw from the study with no obligation and theconfidentiality will be ensured.
Consent for publication
Verbal consent, in the information sheet described the study details, aim for disseminate the finding. Verbal consent was obtained from the participants after explaining the study. Information sheet was given to all participants, who agree to join the study, and met the inclusion criteria, they were approached by one of the researches team.
Availability of data and materials statement
Code numbers was used to identify participants. The hard copy data kept in the
Primary investigator office and stored in a locked cabinet which is only be accessible by primary investigator. Electronic data saved on a password protected computer and PI only authorized to access the data
Availability of research materials
Code numbers was used to identify participants. The hard copy data kept in the
Primary investigator office and stored in a locked cabinet which is only be accessible by primary investigator. Electronic data saved on a password protected computer and PI only authorized to access the data
Authors reporting experiments on humans and/or the use of human tissue samples
There was no bio-specimens or sample collection in this study, therefore this part is not applicable, However, the verbal informed consent was for cognitive testing phase focus group and participant’s interview. This study is not a clinical trial, but it is a POS tool translation into Arabic and validation study
Competing interests
No conflict of interest.
Funding
This research was not funded by any organization
Authors' contributions
Asma: focus group – write the research protocol, data collection, data analysis, review research translation steps and review translated final document and write and review the final manuscript.
Dr. Jessie: facilitate the translation with experts, and write and review the final manuscript.
Zeinab Idris: data collection, write the manuscript, review research translation steps and review translated final document.
Dr. Azza Hassan:review the final manuscript and supervised the research process.
Shaikhah Al Keldi: Data collection, review research translation steps and review translated final document.
Nima Ali: review research translation steps and review translated final document.
Khadra Sofi: review research translation steps and review translated final document.
Ayman Allam: analyses statistic data, write the discussion of manuscript, and review the final manuscript
Acknowledgements
We acknowledge the support of the Medical research committee at Hamad medical Corporation.
References
1. Al Bulushi AM, Critchley KA. Evaluation of Quality of Life Instruments in a Palliative Care Context: An Integrative Literature Review. Middle East J Nursing. 2015;101(2081):1-7.
2. Dakessian Sailian S, Salifu Y, Saad R, Preston N. Dignity of patients with palliative needs in the Middle East: an integrative review. BMC palliative care. 2021;20(1):1-8
3. Collins ES, Witt J, Bausewein C, Daveson BA, Higginson IJ, Murtagh FE. A systematic review of the use of the palliative care outcome scale and the support team assessment schedule in palliative care. Journal of Pain and Symptom Management. 2015;50(6):842-53.
4. Azhar A, Bruera E. Outcome measurement and complex physical, psychosocial and spiritual experiences of death and dying. Ann Palliat Med. 2018;7(Suppl 3):S231-43.
5. Sirati Nir M, Rassouli M, Ebadi A, Moosavi S, Pakseresht M, Hasan Shiri F, et al. Psychometric properties of the Persian version of palliative care outcome scale (POS) in adult patients with cancer. Frontiers in Psychol. 2022;13:858684.
6. Murtagh FE, Ramsenthaler C, Firth A, Groeneveld EI, Lovell N, Simon ST, et al. A brief, patient-and proxy-reported outcome measure in advanced illness: Validity, reliability and responsiveness of the Integrated Palliative care Outcome Scale (IPOS). Palliative med. 2019;33(8):1045-57.
7. Antunes B, Daveson B, Ramsenthaler C, Benalia H, Ferreira P, Bausewein C, et al. The Palliative care Outcome Scale (POS) Manual for cross-cultural adaptation and psychometric validation. London: Cicely Saunders Institute. 2012.
8. Hearn J, Higginson IJ. Development and validation of a core outcome measure for palliative care: the palliative care outcome scale. Palliative Care Core Audit Project Advisory Group. BMJ Quality & Safety. 1999 Dec 1;8(4):219-27.
9. Veronese S, Rabitti E, Costantini M, Valle A, Higginson I.Translation and cognitive testing of the Italian Integrated Palliative Outcome Scale (IPOS) among patients and healthcare professionals. PLoS One. 14(1), e0208536.
10. Martinsson L, Sahlén KG. Translation and cultural adaptation of the Integrated Palliative care Outcome Scale for Dementia (IPOS-Dem) to Swedish. BMC nursing. 2022 Apr 2;21(1):78.
11. Spichiger F, Keller Senn A, Volken T, Larkin P, Koppitz A. Integrated Palliative Outcome Scale for People with Dementia: easy language adaption and translation. J Patient-Reported Outcomes. 2022 Feb 15;6(1):14.
Received: June 05, 2023
Accepted: June 28, 2023
Published: June 30, 2023.
To cite this article : Al Bulushi AM, Johnson J, Idris Z, Hassan AAI, Ali N, Al keldi SM, et al. Arabic Modified Version of Palliative Outcome Scale (POS): Cross Cultural Adaptation and Validation. British Journal of Cancer Research. 2023; 6(1): 629- 634. doi: 10.31488/bjcr.185.
© The Author(s) 2023. This is an open access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/).
