Research article / Open Access
DOI: 10.31488/bjcr.199
Bleeding of the Latest Generation Silicone Breast Implants Very Shortly after Implantation
Evert van den Broek1,2, Rita Kappel*3
1. Department of Pathology, Isala Klinieken, Zwolle, The Netherlands
2. Department of Pathology, University Medical Center Groningen, Groningen, The Netherlands
3. Plastic and reconstructive surgeon, Dr. Kappel Institute, Zwolle, The Netherlands
*Corresponding author: Dr. Rita Kappel MD; Ph.D. Dr. Kappel Instituut / Willemskade 14, 8011 AD Zwolle, The Netherlands, Tel: +31-384225755
Abstract
For decades it has been known that silicone breast implants bleed silicone molecules into the periprosthetic tissue and these are subsequently able to migrate to multiple organs. Data are emerging that silicone gel bleeding is associated with breast implant illness (BII). From the third-generation silicone breast implants in the nineties onwards, a Bleed Retardation Layer (BRL) was added to the silicone elastomer shell as a barrier, in order to retard bleeding of the silicone molecules into the surrounding tissue.This study aims to investigate whether silicone gel bleeding of the newest silicone breast implants can be detected early on after implantation. For that purpose specifically women with a short time of implantation were included in this study, which is unique. As part of the surgical explantation procedure, periprosthetic tissue from a series of 7 women who had breast implant removal early after implantation (range 3 weeks to 28 months) was examined for silicone deposits by conventional light microscopy and stained by Modified Oil Red O (MORO). Surprisingly, silicone deposits were found in periprosthetic tissue of all 7 cases, even in tissue removed within a month after implantation. These data demonstrate that silicone gel bleeding starts very shortly after implantation of the latest generation silicone breast implants. .
Key words: Silicone breast implants, silicone gel bleed.
Introduction
Implementation of silicone breast implants for augmentation and reconstruction has been common practice for decades since the nineteen sixties. It became clear in the seventies that the elastomer shell of silicone breast implants is very permeable to the lower molecular weight fractions of silicone oils- and gels used in the manufacturing of silicone breast implants. Therefore, Brody introduced the term “gel bleed” in 1977 [1]. These medical grade silicone materials were considered to be biocompatible. He was soon followed by Barker et al., who demonstrated the bleeding from bag-gel breast implants and the relationship to the fibrous reaction in 1978 [2]. This was also confirmed by others [3-6]. More recently, evidence is growing that there is a relationship between silicone breast implants and health complaints, described as breast implant illness (BII) [7-15]. In 2016, a case of a deceased woman was described who had silicone breast implants for 17 years. In this study silicone deposits were found in all investigated tissues, including the brain and spinal cord, and revealed massive amounts of Si-atoms per 0.1 µm² of tissue, which appeared to be much more than in an adult without breast implants [16]. It was assumed that silicone bleeding was due to ‘old type-breast implants’ without an added Bleed Retardation Layer (BRL). On the other hand, at almost every explantation operation reduction of volume of the explanted implants is noticed by the surgeon. It is currently unknown when the gel bleed process starts. The aim of this study was to investigate, whether silicone material can already be detected in periprosthetic tissue of women who requested an early explantation of their silicone breast implants. .
Methods
In the period between 2018-2021, all seven consecutive women presented by Dr Kappel Institute (Zwolle, The Netherlands), who requested an early explantation of their silicone breast implants, were included in this study. Of all these women the duration of implantation was less than 2,5 years. Moreover, 3 of them had them for only 3 weeks. The explantation operation was at the Dr Kappel Institute. As part of the standard surgical procedure of opening the capsule, a small sample of periprosthetic tissue was taken for pathological examination. The technical markings, such as lot and serial numbers, were noted in the surgical report. Archival formalin-fixed paraffin-embedded (FFPE) periprosthetic material of these 7 cases was used in compliance with the ‘Code for Proper Secondary Use of Human Tissue’ in The Netherlands (www.federa.org). To assess whether silicone material was present in this tissue, standard HE slides were created. In addition, Modified Oil Red O (MORO) stains were acquired to confirm that the revealed vacuoles in HE stained tissue were silicone material [16]. The HE and MORO stained slides were examined by the pathologist. These findings were matched with clinical data (i.e., age, date of implantation, date of explantation and characteristics of the breast implant). .
Results
All seven women, with a mean age of 32 years, had bilateral silicone breast implants. None of these women had health complaints but requested their explantation because they were unhappy with the augmentation, which was performed elsewhere. All breast implants were intact. The longest implantation time was 28 months, the shortest 3 weeks (n=3). There were 4 patients with cohesive implants, and 3 with round and smooth implants. All cohesive implants were from the Mentor brand. One of the round implants was a Mentor implant, which was a finely textured, almost smooth implant. The other two smooth and round implants were from the Motiva brand. All these 7 implants are modern brands with an international distribution. The results are summarized in Table 1. Standard HE stained slides of resected periprosthetic tissue FFPE samples were obtained from all 7 cases. The investigated samples were all relatively small samples from tissue adjacent to the intact silicone breast implants as part of the procedure of opening the capsule. As expected, all samples show collagen-rich paucicellular fibrosis, according to fibrous capsular formation. A layer of mononuclear cells, largely consisting of macrophages, was also found lining the fibrous capsule. In all these investigated samples, vacuolated macrophages were found to a greater or lesser extent. The standard HE stained slides were examined by conventional light microscopy. The phagocytized vacuoles were characterized by amorph translucent material, indicating deposition of silicone material. Evaluation of additional Modified Oil Red O (MORO) stained slides confirmed the presence of silicone material in the vacuolated cytoplasm of these macrophages [16], since it stained positive in all seven cases. Representative images of HE and corresponding MORO slides are shown in figure 1. .
Table 1. Overview of Cases with Characteristics of the Explanted Breast Implants
| Case | Implantation date | Explantation date | Implantation timelapse | Implant Brand | Lot number | Serial number | Additional information |
|---|---|---|---|---|---|---|---|
| 1 | 2016 | 2018 | 28 months | Mentor 380 cc | 7282628 (2x) | 006 & 007 | Cohesive |
| 2 | 2018 | 2019 | 16 months | Mentor 300 cc | 324 (2x) | 7528136 (2x) | Cohesive microtexture |
| 3 | 2019 | 2020 | 15 months | Motiva 450 cc | 19060434 (2x) | no data | Round, smooth |
| 4 | 2019 | 2020 | 3 months | Mentor 345 cc | 3341152 (2x) | 9365889 (2x) | Cohesive, textured |
| 5 | 2021 | 2021 | 3,5 weeks | Mentor 330 cc | 3341205 (2x) | 9531177 (2x) | Cohesive, fine textured |
| 6 | 2020 | 2020 | 3 weeks | Mentor 325 cc | 3445440 & 3446418 | no data | Round, fine textured |
| 7 | 2021 | 2021 | 3 weeks | Motiva 300 cc | 21050574 (2x) | RSD 300 (2x) | Round, smooth |
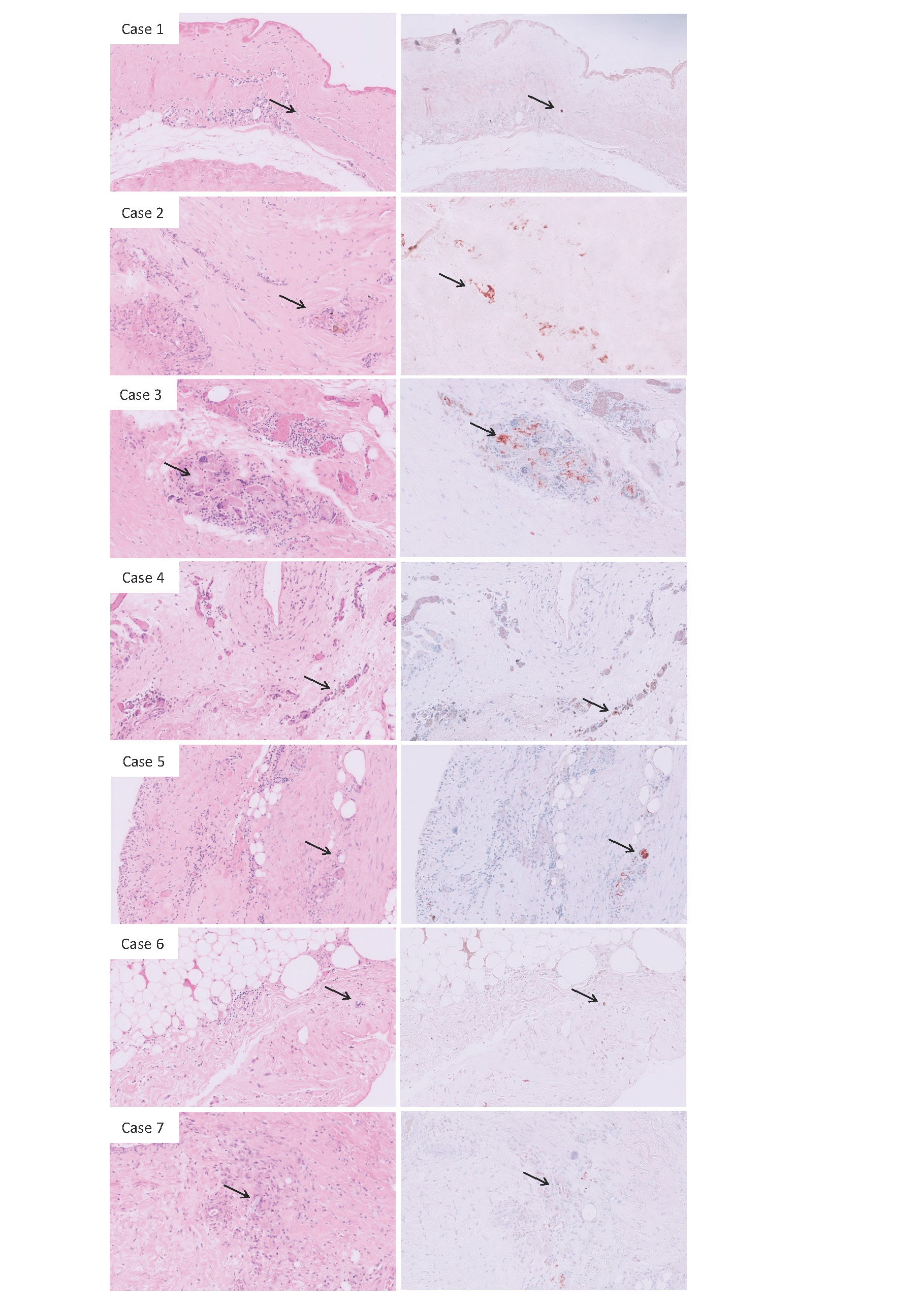
Figure 1:Representative FFPE samples of periprosthetic tissue of 7 cases stained by Hematoxylin and Eosin (left panel) and Modified Oil Red O (right panel). The images show a fibrous capsule with on the side of the breast implant, in varying degrees deposition of eosinophilic fibrinoid material. Furthermore, mononuclear histiocytic cells were seen. In all these 7 cases (A-G) macrophages with intracytoplasmic vacuoles were found containing amorph translucent material denoting deposition of silicones, indicated by arrows. Corresponding MORO-stained slides in order to confirm that this material regards to silicone depositions, show positive, mostly granular staining and were depicted in the right panel (A-G).
Discussion
In this study, a total of 7 women were explanted very shortly after implantation of the latest modern silicone breast implants of widely used reputable brands between 2018 and 2021. The silicone breast implants were removed between 3 weeks and 2,5 years after implantation. These rare cases provide the unique opportunity to assess whether silicone material can already be detected in the periprosthetic tissue of women who requested an early explantation of their silicone breast implants. Despite being the latest generation of silicone breast implants, silicone deposits were found in periprosthetic tissue of all 7 cases. These data demonstrate that silicone gel bleeding starts very shortly after implantation, which was not described earlier. Silicone deposits were not expected to be found in a relatively small sample of periprosthetic tissue so early after implantation in patients with silicone breast implants of the latest generation. Silicone bleeding is an inherent spontaneous process caused by the physico-chemical characteristics of the siloxane building block used to manufacture all silicone breast implants [17,18]. In addition, Batich et al [5] demonstrated in 1996 that the bleeding silicone polymers further disintegrate into smaller fractions, which will increase their total surface area. Moreover, these smaller fractions will be either lipophilic or hydrophilic as a result of reactions of oxidation, hydrolysis and addition. Therefore, they would be capable of interacting with other lipophilic or hydrophilic molecules in their vicinity. The continuous bleeding would result in loss of tensile strength of the elastomer shell of the silicone implant, even to the point of a spontaneous rupture, as these molecules behave as softeners [19]. In addition this process will ultimately result in high amounts of silicon atoms in multiple organs and tissues, as was previously demonstrated by EDX-analysis [16]. While the number of silicone atoms in individuals without silicone breast implants is less than 500 per 1-0,15 square µm, the counts in a woman with silicone breast implants for 17 years were much higher. For instance, 22.211 Si-counts per 0,1-0,15 square µm were measured in the thoracic spinal cord and 7.200 Si-counts per 0,1-0,15 quare µm were found in the thyroid and not less than 108.000 Si-counts per 0,1-0,15 square µm were found in the periprosthetic capsule [16]. The biological consequences of this overabundance of silicone molecules and atoms in several tissues in the body of women who have had silicone breast implants for many years, and how it is related to the development of health complaints, is still unexplained. The current study, demonstrating that silicone bleeding starts instantaneously after implantation of silicone breast implants, emphasizes the need to gain further insight into the molecular process of gel bleeding in relation to breast implant illness (BII). .
Conclusion
In conclusion, from this study it is obvious that the latest generation of silicone breast implants of widely used reputable brands and types, bleed synthetic silicone polymer molecules through the intact elastomer shell. It demonstrates that the bleeding starts instantaneously after implantation and apparently there is no escape from this process. .
Acknowledgments
The authors thank Peter Bult, PhD, MD (Radboud University Medical Centre, Nijmegen, The Netherlands) for his expert assistance in providing HE and MORO-stained slides. .
Competing Interests
None.
Funding
None.
References
1. Brody GS, et al. Fact and fiction about breast implant "bleed". Plast Reconstr Surg. 1977; 60: 615 - 616. .
2. Barker DE, Retsky MI, Schultz S, et al. "Bleeding" of silicone from bag-gel breast implants, and its clinical relation to fibrous capsule reaction. Plast Reconstr Surg. 1978; 61: 836 -841. .
3. Truong LD, Cartwright JJr, Goodman MD, Woznicki D, et al. Silicone lymphadenopathy associated with augmentation mammaplasty. Morphologic features of nine cases. Am J Surg Pathol. 1988; 12: 484 -491. .
4. Garrido L, Pfleiderer B, Jenkins BG, Hulka CA, Kopans DB, et al. Migration and chemical modification of silicone in women with breast prostheses. Magn Reson Med. 1994; 31: 328- 330. .
5. Batich C, DePalma D, Marotta J, Latorre G, et al. Silicone degradation reactions. Curr Top Microbiol Immunol. 1996; 210: 13 -23 (1996). .
6. Beekman W, Feitz R, van Diest PJ, Hage JJ, et al. Migration of silicone through the fibrous capsules of mammary prosthes,es. Ann Plast Surg. 1997; 38: 441- 445. .
7. Solomon G, et al. A clinical and laboratory profile of symptomatic women with silicone breast implants. Semin Arthritis Rheum. 1994; 24: 29 -37. .
8. Melmed EP, et al. A review of explantation in 240 symptomatic women: a description of explantation and capsulectomy with reconstruction using a periareolar technique. Plast Reconstr Surg. 1998; 101: 1364 -1373. .
9. Brawer AE, et al. Amelioration of Systemic Disease after Removal of Silicone Gel-filled Breast Implants, J Nutr Environ Med. 2000; 10, 125 -132. .
10. Brown SL, Pennello G, Berg WA, Soo MS, Middleton MS, et al. Silicone gel breast implant rupture, extracapsular silicone, and health status in a population of women. J Rheumatol. 2001; 28, 996 -1003. .
11. Vermeulen RC, Scholte HR, et al. Rupture of silicone gel breast implants and symptoms of pain and fatigue. J Rheumatol. 2003; 30: 2263 -2267. .
12. Chan SA, Malik F, Wharton S, Klocke R, et al. Systemic inflammatory disease resolution following cosmetic silicone breast implant removal. BMJ Case Rep. 2015; bcr2014207418. .
13. Brawer A, et al. Is Silicone Breast Implant Toxicity an Extreme Form of a More Generalized Toxicity Adversely Affecting the Population as a Whole? Int Ann Med. 2017; 1: 1-7. .
14. Kappel RM, Pruijn GJM, et al. Explantation of Silicone Breast Implants Ameliorates Gel Bleed Related Health Complaints in Women with Breast Implant Illness. Clin Med Rev Case Rep. 2020; 7: 1-7. .
15.Onnekink C, Kappel RM, Boelens WC, Pruijn GJM, et al. Low molecular weight silicones induce cell death in cultured cells. Sci Rep. 2020; 10: 9558. .
16. Kappel RM, Boer LL, Dijkman H, et al. Gel Bleed and Rupture of Silicone Breast Implants Investigated by Light-, Electron Microscopy and Energy Dispersive X-ray Analysis of Internal Organs and Nervous Tissue. Clin Med Rev Case Rep. 2016; 3: 1-9. .
17. LeVier RR, Harrison MC, Cook RR, Lane TH, et al. What is silicone? Plast Reconstr Surg. 1993; 92: 163 -167. .
18. Kappel RM, Klunder AJH, Pruijn GJM, et al. Silicon chemistry and silicone breast implants. Eur J Plast Surg. 2014; 37:123 -128. .
19. Reams BD Jr, et al. Silicone gel-filled breast implants [transcript]. Panel meeting FDA. General and Plastic Surgery Devices Panel. William SH & Co. 1992; 68 - 91.
Received: August 05, 2024;
Accepted: August 27, 2024;
Published: August 30, 2024.
To cite this article : Evert van den Broek, Rita Kappel. Bleeding of the Latest Generation Silicone Breast Implants Very Shortly after Implantation. British Journal of Cancer Research. 2024; 7(3): 732- 735. doi: 10.31488/bjcr.199.
© The Author(s) 2024.
