Research article / Open Access
DOI: 10.31488/bjcr.169
Breast Cancer Susceptibility Genes and Sporadic Tumors: Same Prognosis or Survivorship Bias?
Ballatore Zelmira*1, Bracci Raffaella2, Bianchi Francesca1, Maccaroni Elena1, Bini Federica1, Sonia Crocetti1, Belvederesi Laura1, Brugiati Cristiana1, Murrone Alberto1, Pagliaretta Silvia1, Berardi Rossana1
1. Clinica Oncologica e Centro di Riferimento Regionale di Genetica Oncologica, Università Politecnica delle Marche, AOU Ospedali Riuniti di Ancona – Ancona, Italy
2. Oncologia, ISS - Istituto per la Sicurezza Sociale di San Marino– Cailungo, Repubblica di San Marino
*Corresponding author: Zelmira Ballatore, MD, Clinical Oncology, Polytechnic University of Marche, AOU Ospedali Riuniti, Via Conca 71, 60126 Ancona, Italy.
Abstract
Purpose: Germline BReast Cancer susceptibility genes (BRCA) mutations are found in approximately 5% of all breast cancers (BCs). In the literature there are not univocal data concerning the prognosis of BC in mutation carriers. Aims of this study are comparing outcome among BRCA mutations carriers’ vs not in patients eligible for genetic testing, and investigating relationship between BRCA mutations and main standardized prognostic factors. Methods: Pathologic and clinical features were recorded in all consecutive women with BC referred to perform genetic counseling at our Institution between 2000 and 2019 which resulted eligible for BRCA genetic testing. Results: A total of 485 patients were included, 160 (32.9%) hosted BRCA pathogenic mutation. BRCA related tumors had higher Ki67 and grading than WT ones (p=0.001). There were no differences in relapse free survival between WT, BRCA1 and BRCA2 patients (p=0.96 and p=0.91, respectively). No difference in overall survival between BRCA1 carriers and wt at 10 years (p=0.44) from diagnosis or later (p=0.38) was detected. Conversely, in the first 10 years, BRCA2 tumours reported worse prognostic trend (p=0.044), which was lost later (p=0.10). Conclusion: These results could be influenced by confounding factors like survivorship bias. There seem to be outcome differences only if we consider short term follow up.
Keywords: hereditary breast cancer, BRCA genes, prognostic factors, genetic counselling
Introduction
Hereditary breast cancers (BC) account is estimated for about 5-10% of all BC cases in Western Countries and in up to 20–25% of tumors in patients with a family history of breast and/or ovarian cancer [1,2]. Two major susceptibility genes have been identified to date: BReast Cancer susceptibility gene BRCA1 (17q 12-21) and BReast Cancer susceptibility gene BRCA2 (13q 12-13).
Since their cloning, these genes have been indicated as responsible for breast and ovarian cancer occurrence in high risk families. They are inherited in an autosomal-dominant pattern and confer an increased risk from 40% to 80% for developing BC by age 70. Carriers of germ-line BRCA1/2 mutations also have an increased risk of developing other malignancies, albeit to a lesser extent than BC. An increased frequency of ovarian, prostate, and colon cancer has been reported for BRCA1 carriers, while male BC, pancreatic cancer, ovarian cancer, and some other cancer sites are more frequently observed in BRCA2 carriers [3-6].
BRCA1 positive subjects develop tumors of higher grade and proliferation index, with lower estrogenic receptor levels than patients without mutation [1,7-9]. Conversely, BRCA2 related tumors present pathologic features similar to sporadic disease [1,7-11]. Theoretically prognosis between BRCA-related BC and sporadic one may differ due to the high incidence of adverse tumor features in BRCA-diseases [2,12,13], but even if more than 20 studies investigated this topic results are controversial [1,7-15].
Based on the existence of a Regional Centre of genetic oncology in our University Hospital since mid-nineties, we recorded all BRCA tested patients. Aims of the present study were to compare outcome among BRCA wild type and BRCA mutated BC patients as part of the Hereditary Breast Ovarian Cancer Syndrome (HBOCs), and to investigate relationship between BRCA mutations occurrence and main standardized prognostic factors.
Patients and Methods
Study population
This is a retrospective study and we included all consecutive BC patients referred between January 2000 and June 2019 to our Centre to perform genetic counseling and found eligible for BRCA genetic testing. Patients were offered a counseling program. Male patients were excluded and patients who carried Variants of Unknown Significance (VUS) of BRCA1/2 were excluded. We collected personal and familiar history in family pedigree including three generations and collateral to the third degree of kinship. The genetic test was offered to all patients who had one of the criteria described in Table 1. Patients provided written informed consent to perform the test and to personal data processing, ensuring the confidentiality and direct agreement of use of such informations for scientific research. The counselling protocol was approved by the Ethics Committee of the Marche Region (CERM). The results of the test were returned during a further counseling session. Patients harbouring pathogenic mutations were encouraged to discuss test results with their families. Mutation carriers were offered psychological support as well as a breast/ovary screening program or prophylactic surgery.
Table 1:Criteria apply to selection probands to genetic test
| Selection criteria to genetic test |
| 1. Three First-degree relatives with breast cancer regardless of age |
| 2. Two First-degree relatives with breast cancer younger than 50 years old |
| 3. Two Relatives with bilateral breast cancer regardless of age |
| 4. Two First-degree relatives, one with breast cancer, the other with ovarian cancer at any age * |
| 5. Two First-degree relatives with ovarian cancer *, at any age |
| 6. A Case of breast cancer ≤ 30 years old, even in the absence of familiarity |
| 7. A case of double cancer in the same woman (breast plus ovarian *) regardless of age- |
| 8. A case of male breast cancer even in the absence of familiarity and regardless of age |
| In the last 3 years criteria were extended to include: |
| 9. A Case of breast "triple negative cancer" ≤50 years old |
| 10. A Case of non-mucinous ovarian (or tuba or peritoneal) cancer, even in the absence of familiarity |
Clinical data collection
We collected clinical and prognostic data such as pathological stage, histology, biological features, surgery, medical treatment and events, such as local and/or distant recurrence, second cancers and the date of last observations.
DNA testing
The entire coding sequences of BRCA1 and BRCA2, including flanking intronic regions, were individually PCR amplified from genomic DNA and were purified from peripheral blood leucocytes using Flexigene DNA Blood Mini Kit (Qiagen, Hilden, Germany) according to the instructions of the manufacturer. BRCA1 and BRCA2 sequence was studied using a combination of four techniques namely Direct Sequencing (DS) and Multiple Ligation Probe Amplification (MLPA) analysis.
Direct sequencing
The identification of DNA sequence variants was examined with PCR amplified, as described above, using genomic DNA purified from a separate blood sample. PCR products were purified using the QIAquick® PCR purification kit (Qiagen, Hilden, Germany) filters and sequenced using an automated ABI PRISM 3500DX Sequencing Apparatus. Sequencing reactions were carried out using the ABI PRISM Big Dye Terminator Cycle Sequencing Ready Reaction Kit 3.1 (Applera, Foster City, CA) according to the protocol suggested by the manufacturer. Primer sequences are available from the corresponding Author upon request.
MLPA analysis
MLPA was performed with 200 ng of normal and tumour DNAs using the MRC-Holland BRCA1 and BRCA2 probe kits (Amsterdam, Holland), according to the supplier’s protocol. One l of the FAM-labelled PCR product was then mixed with 1l of fluorescent GeneScan 500 LIZ size standard (Applera) in 15 l of HiDi Formamide, run on an automatic ABI3100 DNA analyzer, and evaluated with GeneScan Software (Applera). The electropherograms showed specific peaks corresponding to each exon of BRCA1 or BRCA2, as well as additional peaks corresponding to control sequences mapping on different chromosomes. A 40-55% decrease of the area of a BRCA1 or BRCA2 exon peak compared to the wild-type control samples was considered as indicative of a heterozygous deletion of that exon 16.
Statistical analysis
According to genetic test outcome, sample population was stratified in three groups: BRCA1 mutated, BRCA2 mutated and BRCA wild-type. For each patient, overall survival (OS) was calculated from the time of diagnosis to the time of death or last follow up visit; relapse free survival (RFS) was calculated from the time of diagnosis to first disease relapse in the HBOCs (bone and/or visceral metastases, second breast cancer diagnosis, local recurrence, ovarian cancer), death from any cause or to the date of last follow-up if none of the preceding events occurred. Survival distribution was estimated by the Kaplan Meier method.
Distribution of the detected data in the three patient groups (BRCA1, BRCA2 and wild type) was compared using log-rank test. The association between categorical variables (clinical, demographic and histopathological features, medical data) was compared in population groups (BRCA1, BRCA2, wild type) and estimated by Chi-Square test. The Cox multivariate proportional hazard regression model was used to assess impact of prognostic factors on survival. Significant differences in survival probability were evaluated by log-rank test. Hazard ratios and 95% confidence intervals (CIs) were estimated from regression coefficients. A significance level of 0.05 was chosen to assess the statistical significance. Statistical analysis was performed with MedCalc package (MedCalc®V16.4.3).
Results
From January 2000 to June 2019, a total of 629 invasive BC patients performed genetic test and of these, 485 female patients were included in the present study. We excluded male subjects (38), 94 women who tested positive for Variants of Unknown Significance (VUS, 40 and 54, BRCA1 and BRCA2 respectively) and 12 patients for which medical history and survival data were not available. A total of 160 women were BRCA carriers, 84 (52.5%) had BRCA1 mutations, while 76 (47.5%) BRCA2. The most frequent detected pathogenic mutations were frameshift (50.0%), nonsense mutations (21.3%), missense (15.0%), rearrangements (9.4%) and splicing site alterations (3.7%) as shown in Table 2.
Table 2:Distribution of detected pathogenetic mutation
| Case Number | ||
|---|---|---|
| Pathogenetic mutations | BRCA1 (%) | BRCA2 (%) |
| Frameshift | 38 (45.2) | 42 (55.3) |
| Missense | 20 (23.8) | 4 (5.3) |
| Rearrangements | 15 (17.9) | 0 (0.0) |
| Splicing site | 5 (6.0) | 1 (1.3) |
| non -sense | 6 (7.1) | 28 (36.8) |
| Silent mutation | 0 (0.0) | 1 (1.3) |
| Total | 84 (100%) | 76 (100%) |
Patients’ characteristics were differentamong the three groups. At diagnosis, the average age was 45.9 years (range 18.3-84.4), 254 patients (52.3%) developed the disease before, while the remaining 231 (47.7%) developed the malignancy later, 327 patients (67.4%) were premenopausal and 155 (32.0%) postmenopausal. Two hundred eighty two women (58.1%) received conservative surgery and 161 (33.2%) underwent simple or radical mastectomy. Table 3 summarizes patients' features.
Follow up and relapse data
At a median follow-up of 7.4 years from diagnosis (range 12.04 - 42.03 years), 72 patients (14.8%) were deceased and 413 patients (85.2%) were still alive. 95 women (19.6%) experienced disease relapse (bone and/or visceral metastases, 2nd breast cancer, contralateral or ipsilateral local recurrence, or ovarian cancer) as shown in Table 3.
Table 3:Clinical and demographic features and relation with BRCA mutation status
| Features | Total No of pts(%) |
Uncarrier No of pts(%) |
BRCA1 mut No of pts(%) |
BRCA2 mut No of pts(%) |
p |
|---|---|---|---|---|---|
| Total No of pts(%) Average age at diagnosis (range years) |
485(100) 45.9(18.3-84.4) |
325(66.9) 47.3(25.4-84.4) |
84(17.3) 42.9(22.1-81.8) |
76(15.5) 46.1(18.3-73.8) |
|
| Age ≤45 years >45years |
254(52.3) 231(47.7) |
152(46.8) 173(53.2) |
56(66.7) 28(33.3) |
46(60.5) 30(39.5) |
0.003 |
| Paus alstatus pre post NA |
327(67.4) 155(32.0) 3(0.6) |
207(63.6) 115(35.5) 3(0.9) |
65(77.4) 19(22.6) 0(0.0) |
55(72.4) 21(27.6) 0(0.0) |
0.053 |
| Second diagnosis cancer Ovarian/breast other solid tumour NA |
89(18.3) 23(4.7) 373(876.9) |
46(14.1) 14(4.3) 265(81.6) |
23(27.4) 7(8.3) 54(64.3) |
20(26.3) 2(2.6) 54(71.1) |
0.21 |
| Ovaro-salpingectomy pre breast cancer diagnosis post breast cancer diagnosis NA |
17(3.5) 28(5.8) 440(90.7) |
10(3.1) 11(3.3) 304(93.6) |
3(3.5) 11(13.1) 70(83.4)? |
4(5.3) 6(7.9) 66(86.8) |
0.47 |
| Prophylactic mastectomy no yes NA |
135(27.8) 24(5.0) 326(67.2) |
110(33.8) 8(2.5) 207(63.7) |
11(13.1) 11(13.1) 62(73.8) |
14(18.4) 5(6.6) 57(75.0) |
<0.001 |
| Relapse of disease* no/unknown yes NA |
367(75.7) 95(19.6) 23(4.7) |
253(77.8) 55(16.9) 17(5.2) |
58(69.0) 21(25.0) 5(6.0) |
56(73.7) 19(25.0) 1(1.3) |
0.17 |
| Deaths no yes |
413(85.2) 72(14.8) |
281(86.5) 44(13.5) |
71(84.5) 13(15.5) |
61(80.3) 15(19.7) |
0.17 |
In all sample, 42.8% of BRCA1 patients (n°36), 38.2% of BRCA2 (n°29) and 35.1% of uncarrier (n°114) received a follow-up longer than 10 years. The mean time between diagnosis and genetic test (Referral Interval, RI) was 5.13 years (range 0.01-37.84 years) and the survival rate resulted similar in the 3 patients groups: 84.5% in BRCA1 patients, 80.3% in BRCA2 and 86.5% in the un-carriers. In the last group 55/325 patients (16.9%) had a relapse of disease: 13 women (23.6%) developed homo or contralateral BC, 10 (18.2 %) were diagnosed with ovarian cancer, 12 (21.8%) developed bone metastases and 10 (18.2%) visceral involvement and finally 10 patients (18.2%) presented local recurrence. In addition there were 2 cases of second cancers (one of angiosarcoma of the breast and one of a colorectal carcinoma). Of all 84 BRCA1 carriers, 21 patients (25.0%) experienced relapse of disease: 10 homo or contralateral BC (47.7%), one of these women also developed ovarian cancer, there were 7 cases (33.3%) of ovarian cancer, 2 patients (9.5%) developed visceral metastasis and 2 (9.5%) local recurrences (9.5%). There was no case of bone relapse. In that group, there was a single case of colorectal cancer. In the group of BRCA2 patients, 19 women (25.0%) reported relapse of disease: 4 homo-contralateral BC cases (21.1%), 4 ovarian cancer cases (21.1%), 6 women (31.6%) developed bone metastases and 4 visceral metastases (21.1%), one case of local recurrence (5.2%).
There were no cases of not related HBOCs tumors. No pancreas case were diagnosed. Mortality due to ovarian cancer was higher in the wild type subgroup than in BRCA1 and BRCA2 (80.0% vs 57.1% and 50.0%, respectively).
Histological characteristics and treatments
In BRCA carriers there was a significant greater proportion of high proliferative and high grade tumors than in wild type one (p = 0.001). In BRCA1 there was a strong association with negative hormonal receptor status (p <0.0001) and stage II-III at the onset (p=0.03). Conversely, more than 60% of BRCA2 and wild type tumors expressed estrogen receptor; BRCA2 tumors presented more frequently lymphovascular invasion (p = 0.018), intraductal component (p = 0.005) and finally presented node involvement in almost 50% of cases (p=0.016) compared to wild type. Her2 positive status was not significantly related to any group as evidenced in Tables 4A and 4B.
Table 4a:Histopatological features and relation to BRCA mutation status
| Features | Total No of pts(%) |
Uncarrier No of pts(%) |
BRCA1 mut No of pts(%) |
BRCA2 mut No of pts(%) |
p |
|---|---|---|---|---|---|
| Stage I II III IV NA |
175(36.1) 164(33.8) 75(15.5) 8(1.6) 63(13.0) |
126(38.8) 106(32.6) 48(14.8) 6(1.8) 39(12.0) |
29(34.5) 35(41.7) 9(10.7) 0(0.0) 12(14.3) |
20(26.3) 23(30.3) 18(23.7) 2(2.6) 13(17.1) |
0.07 |
| Tumour size ≥ 2cm > 2cm NA |
271(55.9) 140(28.9) 74(15.2) |
192(59.1) 84(25.8) 49(15.1) |
41(48.8) 31(36.9) 12(14.3) |
38(50) 25(32.9) 13(17.1) |
0.05 |
| Node status pN0 pN+ NA |
230(47.4) 185(38.2) 70(14.4) |
160(49.2) 122(37.5) 43(13.2) |
43(51.2) 26(31.0) 15(17.9) |
27(35.5) 37(48.7) 12(15.8) |
0.11 |
| Histologic type ductal lobular mixed other NA |
361(74.5) 40(80.2) 16(3.3) 16(3.3) 52(10.7) |
253(77.8) 28(8.6) 12(3.70 12(3.7) 20(6.2) |
63(75.0) 1(1.2) 1(1.2) 3(3.6) 16(19.0) |
45(59.2) 11(14.5) 3(3.9) 1(1.3) 16921.1) |
0.04 |
| Grade low-intermediate(1-2) high(3) NA |
159(32.8) 232(47.8) 94(19.4) |
127(39.1) 133(40.9) 65(20.0) |
10(11.9) 55(64.0) 19(22.1) |
22(28.9) 44(57.9) 10(13.2) |
<0.001 |
| Mib 1 st.Gallen low(<20%) high(≥20%) NA |
105(21.7) 296(61.0) 84(17.3) |
85(26.1) 176(54.2) 64(19.7) |
9(10.7) 64(76.2) 11(12.8) |
11(14.5) 56(73.7) 9(11.8) |
0.001 |
| ER status negative positive NA |
138(28.5) 277(57.1) 70(14.4) |
72(22.2) 202(62.2) 51(15.7) |
56(66.7) 16(19.0) 12(14.3) |
10(13.2) 59(77.6) 7(9.2) |
0.40 |
| Pgr status negative positive NA |
181(37.3) 234(48.2) 70(14.4) |
97(29.8) 177(54.5) 51(15.7) |
63(75.0) 9(10.7) 12(14.3) |
21(27.6) 48(63.2) 7(9.2) |
0.08 |
| Her 2 status negative positive NA |
339(66.9) 70(14.4) 76(15.7) |
218(67.1) 50(15.4) 57917.5) |
63(75.0) 9(10.7) 12(14.3) |
58(76.3) 11(14.5) 7(9.2) |
0.39 |
| Lymphovascular invasion absent/not described focal/massive NA |
255(52.6) 116(23.9) 114(23.5) |
168(51.7) 76(23.4) 81(24.9) |
36(42.9) 15(17.8) 33(39.3) |
23(30.2) 25(32.9) 28(36.9) |
0.02 |
| Necrosis absent/not decribed present NA |
263(54.2) 92(19.0) 130(26.8) |
174(53.5) 60(18.5) 91(28.0) |
26(31.0) 19(22.6) 39(46.4) |
28(36.8) 13(17.1) 35(46.1) |
0.14 |
| Intraductal component absent/not decribed present NA |
175(36.1) 202(41.7) 108(22.3) |
114(35.1) 153(47.1) 58(17.8) |
16(19.1) 18(21.4) 50(59.5) |
6(7.9) 31(40.8) 39(51.3) |
<0.001 |
| Lymphocite infiltration absent/not described present NA |
282(57.9) 47(9.7) 158(32.4) |
196(60.3) 35(10.8) 94(28.9) |
15(17.9) 7(8.3) 62(73.8) |
6(7.9) 5(6.6) 65(85.5) |
0.002 |
| Subtype Luminal A Luminal B Her2- Luminal B Her2+ Her2+ enriched TNBC NA |
73(15.1) 149(30.7) 48(9.9) 24(4.9) 118(24.3) 73(15.1) |
67(20.6) 96(29.5) 34(10.5) 17(5.2) 58(17.8) 53(16.4) |
1(1.2) 11(13.1) 2(2.4) 7(8.3) 52(61.9) 11(13.1) |
5(6.6) 42(53.3) 12(15.8) 0(0.0) 8(10.5) 9(11.8) |
<0.001 |
Table 4b:Treatment and relation to BRCA mutation status
| Features | Total No of pts(%) |
Uncarrier No of pts(%) |
BRCA1 mut No of pts(%) |
BRCA2 mut No of pts(%) |
p |
|---|---|---|---|---|---|
| Surgery Conserving energy mastectomy NA |
282(58.1) 161(33.2) 42(8.7) |
19(60.9) 95(29.3) 32(9.8) |
50(59.5) 29(34.5) 5(6.0) |
34(44.7) 37(48.7) 5(6.6) |
0.003 |
| Sentinel Node no yes NA |
275(56.7) 126(30.0) 84(17.3) |
185(56.9) 101(31.1) 39(12.0) |
45(53.6) 14(16.7) 25(29.7) |
45(59.2) 11(14.5) 20(26.3) |
0.008 |
| Lymphadene nectomy no yes NA |
96(19.9) 329(67.8) 60(12.4) |
81(24.9) 205(63.1) 39912.0) |
9(10.7) 64(76.2) 11(13.1) |
6(7.9) 60(78.9) 10(13.2) |
<0.001 |
| Chemotherapy neo-adjuvant adjuvant none NA |
47(9.7) 207(42.7) 56(11.5) 175(36.1) |
28(8.6) 150(46.2) 51(15.7) 96(29.5) |
10(11.9) 31(36.9) 1(1.2) 42(50.0) |
9(11.8) 26(34.2) 4(5.3) 37(48.7) |
0.09 |
| Adjuvant endocrine therapy no yes NA |
107(22.1) 175(36.1) 203(41.8) |
69(21.2) 137(42.2) 119(36.6) |
33(39.3) 6(7.1) 45(53.6) |
5(6.6) 32(42.1) 39(51.3) |
0.98 |
| Complementary radiotherapy no yes NA |
82(16.8) 186(38.3) 271(55.9) |
59(18.2) 144(44.3) 122(37.5) |
6(7.1) 28(33.3) 50(59.5) |
17(22.4) 14(18.4) 45(59.2) |
0.04 |
Prognostic associations
At univariate analysis, in the mutated BRCA1 group there were no variable showing a prognostic impact on RFS and OS. Conversely, in BRCA2 the prognostic role of small tumor size (p= 0.037) and negative node status (p= 0.021) was confirmed in RFS and the positive impact of early stage was evidenced both in RFS and in OS (p=0.001 and p=0.006, respectively). These last findings were not confirmed at multivariate analysis (Table 5, Table 6, Table 7).
Table 5:Prognostic impact of key tumor characteristic in BRCA1 mutated patients group.
| Features | Univariate Analysis OS | Univariate Analysis RES | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| age,years <45ys >45 |
0.67 | 0.20 to 2.16 | 0.48 | 1.66 | 0.61 to 4.08 | 0.34 |
| Pausal ststus pre vs post |
0.49 | 0.09 to 1.71 | 0.22 | 1.63 | 0.54 to 4.30 | 0.42 |
| Tumors size <2cm >2cm |
0.60 | 0.18 to 1.97 | 0.39 | 0.69 | 0.24 to 1.79 | 0.41 |
| Nodal status pN0 vs pN+ |
0.94 | 0.23 to 3.86 | 0.93 | 0.69 | 0.26 to 1.79 | 0.43 |
| Stage I+IIA VS IIB+III |
2.03 | 0.56 to 7.38 | 0.21 | 1.95 | 0.67 to 5.67 | 0.15 |
| Grade low-intermediate vs high |
0.59 | 0.12 to 3.59 | 0.62 | 1.11 | 0.12 to 10.58 | 0.91 |
| M ib 1 st.Gallen <20% vs ≥20% |
0.64 | 0.12 to 3.87 | 0.67 | 0.61 | 0.19 to 2.25 | 0.50 |
| ER status negative vs positive |
1.39 | 0.34 to 5.38 | 0.66 | 0.55 | 0.21 to 1.69 | 0.33 |
| PeR status negative vs psoitive |
0.73 | 0.13 to 3.83 | 0.67 | 0.29 | 0.13 to 1.54 | 0.20 |
| Her 2 status negative vs positive |
0.55 | 0.07 3.12 | 0.43 | 1.55 | 0.27 to 7.65 | 0.67 |
| Surgery conservine vs mastectomy |
0.66 | 0.20 to 2.08 | 0.47 | 0.68 | 0.27 to 1.64 | 0.37 |
Table 6:Prognostic impact of key characteristic in BRCA 2 mutated patients group.
| Features | Univariate Analysis OS | Univariate Analysis RES | multivariate Analysis RES | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | ||
| age,years <45ys >45 |
1.61 | 0.54 to 4.87 | 0.39 | 0.72 | 0.26 to 1.87 | 0.47 | |
| Pausal ststus pre vs post |
0.88 | 0.2 to 2.92 | 0.82 | 0.72 | 0.27 to 2.44 | 0.72 | |
| Tumors size <2cm >2cm |
0.40 | 0.13 to 1.18 | 0.09 | 0.36 | 0.12 to 0.94 | 0.037 | 0.26 |
| Nodal status pN0 vs pN+ |
0 | 0.05 to 0.57 | <0.001* | 0.25 | 0.12 to 0.84 | 0.021 | 0.20 |
| Stage I+IIA VS IIB+III |
13.56 | 3.50 to 52.42 | 0.001 | 3.99 | 1.36 to 11.68 | 0.006 | 0.09 |
| Grade low-intermediate vs high |
0.53 | 0.15 to 2.18 | 0.42 | 1.47 | 0.54 to 4.23 | 0.42 | |
| M ib 1 st.Gallen <20% vs ≥20% |
0.49 | 0.14 to 2.81 | 0.49 | 1.17 | 0.32 to 4.42 | 0.80 | |
| ER status negative vs positive |
2.67 | 0.65 to 23.51 | 0.14 | 0.86 | 0.23 to 3.18 | 0.81 | |
| PeR status negative vs psoitive |
1.58 | 0.42 to 6.37 | 0.47 | 1.4 | 0.51 to 3.76 | 0.51 | |
| Her 2 status negative vs positive |
0.41 | 0.05 to 1.69 | 0.16 | 1.32 | 0.34 to 4.89 | 0.71 | |
| Lymphovascular invasion absent vs present |
0.67 | 0.15 to 2.9 | 0.59 | 1.12 | 0.37 to 3.95 | 0.75 | |
| Surgery conservine vs mastectomy |
0.7 | 0.21 to 2.34 | 0.57 | 0.47 | 0.18 to 1.26 | 0.14 | |
Table 7:Prognostic impact of key tumour characteristic in BRCA wild type patients group
| Features | Univariate Analysis OS | Multi variate OS |
Univariate Analysis RES | Multi variate RES |
||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | P-value | HR | 95% CI | P-value | P-value | |
| age,years <45ys >45 |
0.88 | 0.48 to 1.62 | 0.68 | 1.08 | 0.68 to 1.71 | 0.74 | ||
| Pausal ststus pre vs post |
0.69 | 0.36 to 1.29 | 0.24 | 0.85 | 0.52 to 1.37 | 0.50 | ||
| Tumors size <2cm >2cm |
0.48 | 0.21 to 0..91 | 0.026 | 0.048 | 0.54 | 0.27 to 0.87 | 0.016 | 0.34 |
| Nodal status pN0 vs pN+ |
0.67 | 0.34 to 1.30 | 0.67 | 0.78 | 0.46 to 1.30 | 0.33 | 0.20 | |
| Stage I+IIA VS IIB+III |
3.69 | 1.61 to 8.12 | <0.001 | <0.001 | 2.45 | 1.29 to 4.68 | <0.001 | 0.03 |
| Grade low-intermediate vs high |
0.40 | 0.19 to 0.86 | 0.018 | 0.22 | 0.57 | 0.32 to 0.98 | 0.046 | 0.32 |
| M ib 1 st.Gallen <20% vs ≥20% |
0.63 | 0.30 to 1.37 | 0.25 | 0.86 | 0.48 to 1.52 | 0.59 | ||
| ER status negative vs positive |
0.47 | 0.18 to 0.90 | 0.027 | 0.40 | 0.46 | 0.19 to 0.72 | 0.003 | <0.001 |
| PeR status negative vs psoitive |
0.83 | 0.39 to 1.71 | 0.83 | 0.68 | 0.36 to 1.17 | 0.15 | ||
| Her 2 status negative vs positive |
0.38 | 0.10 to 0.71 | 0.008 | 0.84 | 0.58 | 0.25 to 1.09 | 0.08 | |
| Lympho vascular invasion absent vs present |
0.69 | 0.28 to 1.58 | 0.35 | 0.56 | 0.24 to 0.96 | 0.039 | 0.26 | |
| Necrosis absent vs present |
1.56 | 0.27 to 1.58 | 0.39 | 1.04 | 0.52 to 2.09 | 0.90 | ||
| Intraductal component absent vs present |
1.74 | 0.87 to 3.51 | 0.11 | 1.063 | 0.61 to 1.83 | 0.84 | ||
| tumor infiltrating lymphocytes absent vs present |
0.51 | 0.13 to 1.29 | 0.13 | 0.90 | 0.39 to 2.08 | 0.80 | ||
| surgery conservine vs mastectomy |
0.69 | 0.34 to 1.34 | 0.26 | 0.59 | 0.33 to 0.95 | 0.032 | 0.63 | |
| Chemotheraphy neo/adjuvant vs no |
2.48 | 1.33 to 4.65 | 0.004 | 0.32 | 0.65 | 0.38 to 1.02 | 0.06 | |
| Adjuvant endocrine therapy ves vs no |
0.48 | 0.18 to 0.97 | 0.04 | 0.87 | 0.51 | 0.24 to 0.85 | 0.014 | 0.001 |
There was no significant difference in OS (HR 1.09, 95% CI 0.68-0.77, p=0.70) and in RFS (HR= 1.06, 95% CI 0.71-1.57, p=0.77) between mutation carriers (BRCA1 plus BRCA2) and BRCA WT patients (Figures 1 and 2) and these results were confirmed also from the analysis of the three distinct subgroups (respectively p = 0.96 and p = 0.91) (Figures 3 and 4).
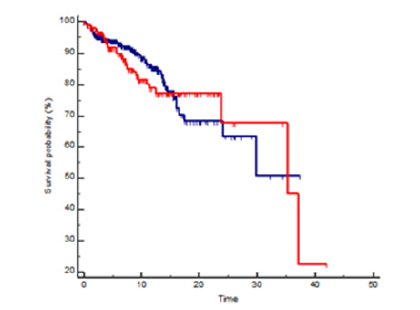
Figure 1:Kaplan Meyer OS probability in BRCA mutated (red line) vs wild type (blue line) patients, HR 1.09 95%CI 0.68-1.77, p=0.70.
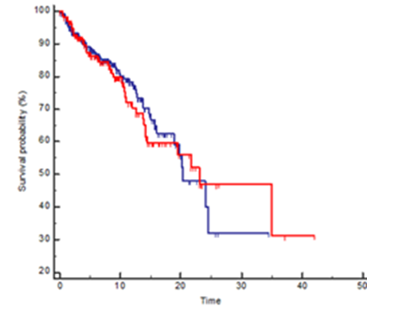
Figure 2:Kaplan Meyer RFS probability in BRCA mutated (red line) vs wild type (blue line) patients, HR 1.06 95%CI 0.71-1.57, p=0.77.
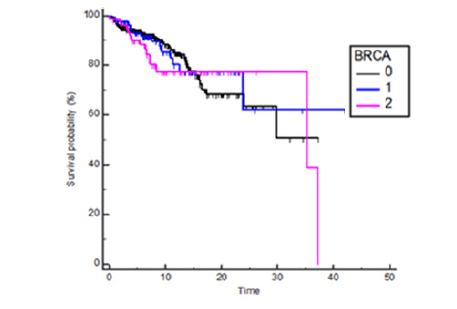
Figure 3:Kaplan Meyers OS probability in BRCA 1 and BRCA 2 vs wild type patients, BRCA1 HR 0.94 95%CI 0.52-1.71, BRCA2 HR1.27 95%IC 0.68-2.39, p= 0.66
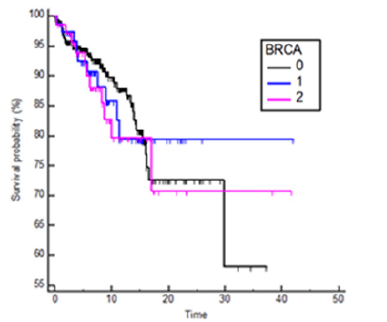
Figure 4:Kaplan Meyers RFS probability in BRCA 1 and BRCA 2 vs wild type patients, BRCA1 HR 1.06 95%CI 0.66-1.72, BRCA2 HR1.03 95%IC 0.61-1.74, p= 0.97
No OS difference was observed between BRCA1 mutation carriers and wild type at 10 years from the onset (HR 1.34 95%IC 0.59- 3.02, p=0.44) or after a longer period (HR 0.62 95%IC 0.24-1.61, p=0.38) (Figure 5). Anyway BRCA2 patients, compared to wild-type subgroup, showed a trend to the limit of statistical significance to a worse prognosis, considering the first 10 years from diagnosis (HR 1.95 95% CI 0.89-4.23, p = 0.044). That trend was lost in the follow up over 10 years (HR 0.33, 95% CI 0.12-0.90, p=0.10) (Figure 6). Among patients which developed ovarian cancer, ovarian cancer-related deaths rate was 23.1% in BRCA1 group (3 deaths out of 8 cases), 13.3% in BRCA2 group (2 deaths out of 4 cases) and 18.1% in wild type one (8 deaths out of 8 cases). Ovarian cancer had the highest lethality in the wild type group (100% of mortality) compared to 57.1% and 50.0% in the BRCA1 and BRCA2 patients, respectively.
Discussion
Following the sequencing of BRCA genes, a growing interest in phenotypic definition of related cancers and the possible association with unfavorable histopathological features led to the hypothesis of different outcomes, but to date literature data showed controversial results [12,16-19]. Understanding of prognostic implications of BRCA mutation status in early BC could influence treatment decisions, prophylactic mastectomy, and screening. Our study was designed to investigate BRCA related BC in female patients and to compare prognostic outcomes between BRCA mutated and wild type subjects. We evaluated whether and how the condition of BRCA susceptibility could be prognostically considered. There were not statistical differences in RFS and OS between BRCA-related BC patients and BRCA-wild type ones. According to literature data, the commonly used prognostic factors do not seem to have a significant impact in BRCA mutated patients, especially in BRCA1 [12,13,20,21]. A possible explanation is that poor prognosis due to these unfavorable features could be attenuated by the administration of aggressive treatment including usually chemotherapy, according to greater chemosensitivity due to the deficient homologous recombination repair capability. Literature data validate the favorable impact of chemotherapy in BRCA1 mutated patients, reporting lower OS in absence of adjuvant treatment compared to wild type patients [13,22]. Moreover, stratifying for stage of disease and biological features, for those patients receiving chemotherapy, survival rates were higher in BRCA1 carriers than in wild type ones [20]. All these assessments confirm the state of the art of ovarian cancer clinical practice in which high chemo-sensitivity implies better outcomes in BRCA positive ovarian cancer patients.
To date, some histological features in BRCA tumors have been poorly investigated [23], but our study evidenced lymphovascular invasion and intraductal component significantly expressed in BRCA2-related tumors (respectively p = 0.002 and p <0.001). Conversely, BRCA1-related carcinoma did not show significant differences compared with WT regarding these two features. In BRCA2 related tumors frequent node involvement and a high stage at diagnosis were evident. That could be explained by the young age of BRCA patients (52.3% under 45 years) that is outside the current mammographic screening range. Moreover, the advanced stage of disease may partly justify that almost 50% of BRCA2 carriers received radical or simple mastectomy. Our study confirmed that BRCA2 related tumours tend to relapse with bone metastases, while in BRCA1 group visceral metastases were more common than bone relapse. Those results are supported by the prevalence of luminal and triple-negative subtypes respectively in these two patient subsets.
At a follow-up up to 10 years, patients with BRCA2 mutation seem to have a worse prognosis than wild type (HR 1.95, 95% CI 0.89-4.23, p = 0.044). A similar result was already described by Goodwin P et al in a prospective cohort study [13]. Similarly to our results, univariate analysis showed a worse prognostic trend in OS for BRCA2 carriers (HR = 1.81, 95% CI = 1.15- 2.86, p = 0.01), which did not reach statistical significance at multivariate analysis. Authors concluded that the possible prognostic differences could be due to a greater proportion of patients who underwent adjuvant chemotherapy in BRCA1 group and to a greater presence of unfavorable tumor features in BRCA2 [13]. In our study BRCA2 mutated tumors show a more frequent lymphovascular involvement compared to wild type and this can partially justify this result. This trend is lost after 10 years when the survival curves seem to overlap. Anyway, in this setting the sample was limited and therefore data could not be conclusive. Patients who developed ovarian cancer had a referral interval for the test that was similar to the RFS. These data underline the existence of a patients group who referred to genetic counseling after a second malignancy diagnosis (ovarian cancer), justifying the high percentage of ovarian cancer cases also in wild type group, suggesting either missed or other increased risk mutations in this cohort
In our study, subgroups survival analysis has allowed interpretation with wider reading frame thanks to consecutive series not only through more than 20 years of genetic counselling activities at our Centre, but also consistent criteria for inclusion of the test, with a detection rate next to the 30% comparable to studies with larger series [24]. In addition, our series is balanced for the distribution of stage at diagnosis, surgical and medical treatments received before the mutation status knowledge, for the population characteristics in the 3 subgroups BRCA1, BCA2, wild-type and for the wide range of follow-up. Finally, the evidence of the overlapping referral interval in all groups implies that survivorship bias is equally distributed. Of course to be tested patients must be alive, and we cannot exclude that some patients with poor prognostic factors and BRCA mutation died relatively early, before testing. The only chance to eliminate the survivorship bias in this very complex prognostic evaluation would be to counsel and test all patients at the time of diagnosis. In our study, wild type group was not made of “sporadic” BC patients, but of BC patients which presented criteria for genetic counselling and test (for example for family history), sharing similar characteristics to BRCA carriers but without mutation. Nonetheless counseling involves also “selection” and “referral with consequent “bias”, which can influence results. But what is the best control group in BRCA counseling selected patients? If we want to choose a control group, we have to keep in mind they have to be consecutive with the same characteristics, in the same period, with the same treatment and they all have to undergo the test and be found wild type. A possible explanation for inconsistency between the different series described in the literature could be different selection criteria for counseling, different percentage of BRCA1 or BRCA2 mutation in the studies, and last but not least different ethnicity or populations (ashkenazi vs not, populations with founder mutation that can have a possible poor or better prognosis). A limitation of our study is the retrospective nature that involves selection bias due to confounding factors not always measurable. Many data are self-reported by patients and the long period of the study led to collect a heterogeneous sample for different selection criteria, different received treatment and follow-up modality given these last 20 years improvements in clinical practice. HBOCs selection criteria are becoming more and more precise and defined [25,26]; for example now triple negative breast cancer in a woman under 60 years is sufficient for testing, such a single case of non-mucinous and non-borderline ovarian cancer.
Conclusion
In our study cohorts, there seem not to besignificant survival difference between BRCA1-BRCA2 and WT tumors. We may assume that some factors like survivorship bias and selection bias (relative to the wild type group) could have influenced this result. Moreover we did not incorporate all BC patients, but only ones referred to genetic counseling, with possible referral bias. Only a difference between BRCA2 and wild type patients within 10 years was observed, with a worst prognosis (OS) for BRCA2 patients. Both groups suffer relapse with second malignancy such as breast and ovarian cancers and this confounds prognosis, although it is known the high chemotherapy sensitivity in the last case of relapse, suggesting other genomic alterations in WT group led to increased malignancies risk. By the way, reaffirming the prognostic impact of prophylactic ovary-salpingectomy in the BRCA positive women reduce the development of both ovarian and second breast cancer. In our series we included patients from the same geographical area, likely to have a common genetic background, this emphasizes the importance to perform genetic test in order to identify individuals who have, constitutionally, a high risk of developing breast cancer as part of HBOCs. The genetic risk awareness is the starting point for preventive surveillance in a setting of patients who become ill in an age group not covered by the usual screening programs.
Abbreviations
"BRCA": Breast Cancer susceptibility genes; "BCs": Breast Cancers; "WT": wild type; “HBOCs”: Hereditary Breast Ovarian Cancer Syndrome; “CERM”: Ethics Committee of the Marche Region; “DNA”: Deoxyribonucleic Acid; “PCR”: polymerase chain reaction; “DS”: Direct Sequencing; “MLPA”: Multiple Ligation Probe Amplification; “OS”: overall survival; “RFS”: relapse free survival; “IC”: confidence interval; “HR”: hazard ratio; “VUS”: Variants of Unknown Significance.
Conflicts of Interest
The authors declare no conflict of interest.
Ethics Approval
“All procedures performed in studies involving human participants” including genetic counseling protocol and testing “were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards” and was approved by Ethics Committee of the Marche Region (CERM).
Consent to Participate
Informed consent was obtained from all individual participants included in the study.
Availability of Data and Material
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Acknowledgements
None.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Authors Contribution
Conceptualization and Design: B.Z., B.R. ; Data Curation: B.Z., B.R., B.Fr., M.E., B. Fe., C.S., B.L., B.C., M. A., P.S., B.Ro. ; Statistical Analysis: B.Z. ; Investigation: B.Z. ; Methodology: B.Z.; Software: B.Z.; Visualization: B.Z., B.Fe; Writing- original draft: B.Z., B.R., B. Fe; Writing- review and editing: B.Z., C.S; Supervision: B.Ro, B.Z.; Validation: B.Z, B. Ro.
References
1. Kirova YM, Savignoni A, Sigal-Zafrani B, et al. Is the breast-conserving treatment with radiotherapy appropriate in BRCA1/2 mutation carriers? Long-term results and review of the literature. Breast Cancer Res Treat. 2010; 120: 119-26.
2. Lee EH, Park SK, Park B, et al. KOHBRA Research Group; Korean Breast Cancer Society. Effect of BRCA1/2 mutation on short-term and long-term breast cancer survival: a systematic review and meta-analysis. Breast Cancer Res Treat. 2010; 122: 11-25
3. Ottini L, D’Amico C, Noviello C, et al. BRCA1 and BRCA2 mutations in central and southern Italian patients. Breast Cancer Res. 2000; 2: 307-10.
4. Chappuis PO, Nethercot V, Foulkes WD. Clinico-pathological characteristics of BRCA1- and BRCA2-related breast cancer. Semin Surg Oncol. 2000; 18: 287-95.
5. Rosner B, Colditz GA, Willett WC. Reproductive risk factors in a prospective study of breast cancer: the Nurses' Health Study. Am J Epidemiol. 1994; 139: 819-35.
6. Kotsopoulos J, Lubinski J, Salmena L, et al. Breastfeeding and the risk of breast cancer in BRCA1 and BRCA2 mutation carriers. Breast Cancer Res. 2012; 14: 42.
7. Bray F, Ferlay J, Soerjomataram I, et al. GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68: 394-424.
8. Fondazione IRCCS Istituto Nazionale dei Tumori. I Tumori in Italia - Sito di Epidemiologia Oncologica S.C. Epidemiologia Analitica e Impatto Sanitario. 2016; http://www.tumori.net/it
9. Youlden DR, Cramb SM, Yip CH, et al. Incidence and mortality of female breast cancer in the Asia-Pacific region. Cancer Biol Med. 2014; 11: 101-15.
10. National Cancer Institute. Cancer types, Breast Cancer. 2018; http://www.cancer.gov/types/breast
11. Collaborative Group on Hormonal Factors in Breast Cancer. Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol. 2012; 13: 1141–1151.
12. Bordeleau L, Panchal S, Goodwin P. Prognosis of BRCA-associated breast cancer: a summary of evidence. Breast Cancer Res Treat. 2010; 119: 13-24.
13. Goodwin PJ, Phillips KA, West DW, et al. Breast cancer prognosis in BRCA1 and BRCA2 mutation carriers: an International Prospective Breast Cancer Family Registry population-based cohort study. J Clin Oncol. 2012; 30: 19-26.
14. Li CI, Uribe DJ, Daling JR. Clinical characteristics of different histologic types of breast cancer. Br J Cancer. 2005; 93: 1046–1052.
15. Chipman J, Drohan B, Blackford A, et al. Providing access to risk prediction tools via the HL7 XML-formatted risk web service. Breast Cancer Res Treat. 2013; 140: 187–93.
16. Maccaroni E, Bracci R, Giampieri R, et al. Prognostic impact of mismatch repair genes germline defects in colorectal cancer patients: are all mutations equal? Oncotarget. 2015; 6: 38737-48.
17. Huszno J, Kołosza Z, Grzybowska E. BRCA1 mutation in breast cancer patients: Analysis of prognostic factors and survival. Oncol Lett. 2019; 17: 1986-1995.
18. DeTalhouet S, Peron J, Vuilleumier A, et al. Clinical outcome of breast cancer in carriers of BRCA1 and BRCA2 mutations according to molecular subtypes. Sci Rep. 2020; 10: 7073.
19. Zhong Q, Peng HL, Zhao X, et al. Effects of BRCA1- and BRCA2-related mutations on ovarian and breast cancer survival: a meta-analysis. Clin Cancer Res. 2015; 21: 211-20.
20. Rennert G, Bisland-Naggan S, Barnett-Griness O, et al. Clinical outcomes of breast cancer in carriers of BRCA1 and BRCA2 mutations. N Engl J Med. 2007; 357: 115-23.
21. Phillips K, Andrulis I, Goodwin PJ. Breast Carcinomas Arising in Carriers of Mutations in BRCA1 or BRCA2: Are They Prognostically Different? J Clin Oncol. 1999; 17: 3653-3663.
22. Huzarski T, Byrski T, Gronwald J, et al. Ten-year survival in patients with BRCA1-negative and BRCA1-positive breast cancer. J Clin Oncol. 2013; 31: 3191-6.
23. Heerma van Voss MR, van der Groep P, Bart J, et al. Lympho-vascular invasion in BRCA related breast cancer compared to sporadic controls. BMC Cancer. 2010; 10: 145.
24. Azzollini J, Scuvera J, Bruno E, et al. Mutation detection rates associated with specific selection criteria for BRCA1/2 testing in 1854 high-risk families: A monocentric Italian study. Eur J Intern Med. 2016; 32: 65-71.
25. Cortesi L, Turchetti D, Marchi I, et al. Breast cancer screening in women at increased risk according to different family histories: an update of the Modena Study Group experience. BMC Cancer. 2006; 6: 210.
26. Esposito A, Criscitiello C, Curigliano G. Highlights from the 14(th) St Gallen International Breast Cancer Conference 2015 in Vienna: Dealing with classification, prognostication, and prediction refinement to personalize the treatment of patients with early breast cancer. Ecancermedicalscience. 2015; 9: 518.
Received: August 10, 2021;
Accepted: August 24, 2021;
Published: August 27, 2021.
To cite this article : Zelmira B, Raffaella B, Francesca B, et al. Breast Cancer Susceptibility Genes and Sporadic Tumors: Same Prognosis or Survivorship Bias?. British Journal of Cancer Research. 2021; 4:2.
©2021 Zelmira B, et al.
