Research Article / Open Access
DOI: 10.31488/bjcr.162
Impact of Empirically Eliminating 5-Fluorouracil Bolus and Leucovorin in Patients with Metastatic Colorectal Cancer Receiving First-Line Treatment with mFOLFOX6
Alexa J. Basilio1,2,*, Anand B. Shah1, Katelyn R. Sommerer1, Sarah S. Chehab1, Salvatore M. Bottiglieri1, Iman Imanirad1
1. H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL, USA
2. Emory Healthcare, Winship Cancer Institute, Atlanta, GA, USA
*Corresponding author:Alexa J. Basilio, PharmD, Clinical Pharmacy Specialist, Medical Oncology, Emory Healthcare, Winship Cancer Institute, 1365 Clifton Road NE Atlanta, Georgia, USA 30322.
Abstract
Purpose: Chemotherapy regimens with a 5-fluorouracil– (5-FU–) based backbone, such as mFOLFOX6, are preferred first-line treatments for patients with metastatic colorectal cancer (mCRC). Providers may empirically eliminate the 5-FU bolus because of hematologic toxicities. We analyzed the effects of empirically eliminating the 5-FU bolus and leucovorin (LV) on mCRC patients receiving first-line treatment with mFOLFOX6. Patients and Methods: This retrospective chart review included patients ≥18 years of age with mCRC who received palliative first-line mFOLFOX6 chemotherapy with or without 5-FU bolus and LV between January 1, 2015 and August 31, 2019 at Moffitt Cancer Center. The primary endpoint was progression-free survival (PFS). Secondary endpoints included overall survival (OS), disease control rate (DCR) at first scan, safety, and use of growth factor support. Results: Out of 619 patients screened, 61 were included in the bolus arm and 72 in the non-bolus arm. No difference was observed in median PFS (8.12 vs 6.64 months; P = 0.787) or OS (29.4 vs 21.6 months; P = 0.395) between the bolus and non-bolus arms. DCR at first scan was similar between both arms (82% vs 86%; P = 0.44). Fewer grade ≥3 treatment-related hematologic adverse events (AEs) were observed in the non-bolus arm (22% vs. 38%; P = 0.058). Use of growth factor support was significantly higher in the bolus arm (23% vs 7%; P = 0.012). Conclusion: There was no difference in PFS, OS, or DCR between the non-bolus and bolus arms. The non-bolus arm required less growth factor support and showed a favorable safety profile. This study provides support for removal of the 5-FU bolus in patients receiving palliative therapy with mFOLFOX6 for mCRC.
Introduction
Colorectal cancer is the third leading cause of cancer-related deaths in the United States, with a 5-year survival rate of 14.2% in the metastatic setting despite significant advances in systemic therapy [1]. 5-fluorouracil (5-FU) based chemotherapy has remained the backbone of systemic therapy for metastatic colorectal cancer (mCRC) for several decades [2]. The addition of oxaliplatin or irinotecan to 5-FU and leucovorin (LV) has shown improved response rates, progression free survival (PFS), and overall survival (OS), and these agents have thus become options for first-line treatment of mCRC [3-5]. At Moffitt Cancer Center (MCC), the preferred regimen for front-line treatment is mFOLFOX6 (5-FU, LV, oxaliplatin) with or without bevacizumab. This regimen contains a 5-FU intravenous (IV) bolus schedule administered as an IV push over 5 minutes and a 5-FU continuous IV infusion schedule administered as a slow IV infusion over 46 hours.
Based on the schedules of administration, 5-FU can exert its cytotoxic effects through 2 different mechanisms of action. First, one of its active metabolites, fluorodeoxyuridine monophosphate (FdUMP), inhibits thymidylate synthase, resulting in the inhibition of DNA synthesis. This mechanism is S-phase specific and is associated with the continuous infusion of 5-FU. The second mechanism of action is the direct incorporation of fluorodeoxyuridine triphosphate (FdUTP) and fluorouridine triphosphate (FUTP) into the DNA and RNA of cancer cells, respectively. This mechanism is associated with high plasma concentrations of 5-FU, as seen with 5-FU bolus administration [6,7]. Furthermore, the toxicity associated with 5-FU may present differently depending on the schedule of administration. The toxicities most commonly observed after the administration of the 5-FU bolus schedule are myelosuppression, diarrhea, and mucositis [6,7]. The increased frequency of myelosuppression is thought to be a result of the higher concentrations of 5-FU in the bone marrow after bolus infusion [8]. However, myelosuppression occurs infrequently; instead, hand-foot syndrome is the toxicity primarily observed after administration of the 5-FU continuous infusion schedule [6,7]. In 1998, the Meta-Analysis Group in Cancer reported significantly lower grade 3/4 hematologic toxicities with continuous 5-FU infusion (4%) compared to 5-FU bolus (31%) in mCRC patients. Continuous 5-FU infusion was also associated with higher tumor response rates and improved median overall survival compared to 5-FU bolus (22% vs 14%; P = 0.0002 and 12.1 vs 11.3 months; P = 0.04, respectively) [9,10].
Some providers may choose to empirically eliminate 5-FU bolus and LV when treating mCRC patients who receive palliative therapy because of hematologic and gastrointestinal toxicities associated with bolus dosing. Eliminating these agents may prevent treatment delays, dose reductions, and the need for growth factor support. In the MCC Gastrointestinal Oncology Program, the decision to include or empirically withhold the 5-FU bolus is variable among providers. Whereas some providers proactively omit 5-FU/LV almost in all circumstances with the initiation of treatment, others take a more reactive approach with dose adjustments implemented based on the emergence of toxicities. Based on previous studies, withholding the 5-FU bolus has the potential to decrease unwanted adverse events. However, the impact of withholding on efficacy and survival outcomes is not clear. In this study, we assessed the impact of empirically eliminating 5-FU bolus and LV from first-line treatment with mFOLFOX6 in mCRC.
Methods
We performed a single-center retrospective analysis of patients with mCRC who received palliative first-line mFOLFOX6 chemotherapy with or without the 5-FU bolus and LV components. Based on our institutional practice, we universally discontinue LV along with 5-FU bolus once the decision is made to eliminate this component of chemotherapy. Patients were identified through a comprehensive list generated using International Classification of Disease (ICD) 9/10 codes for mCRC (C18.0-9: malignant neoplasm of colon; C19: malignant neoplasm of rectosigmoid junction; and C20: malignant neoplasm of rectum).
We included patients who were ≥18 years of age, diagnosed with mCRC, and initiated on palliative first-line mFOLFOX6 ± bevacizumab or anti-epidermal growth factor receptor chemotherapy from January 1, 2015 through August 31, 2019 at MCC. Patients were excluded if they had ≥ 2 concurrent malignancies at the time of mFOLFOX6 initiation or received FOLFOX during a clinical trial. A full list of exclusion criteria can be found in Figure 1. Patients were categorized into 2 groups: those who did receive 5-FU bolus and LV (bolus arm) and those who did not receive 5-FU bolus and LV (non-bolus arm). Data analysis cutoff was December 31, 2019.
The primary endpoint was PFS, which was measured from the date of mFOLFOX6 therapy initiation until the date of earliest documented progression. Progression was measured by means of an imaging study, clinician documentation of progression in the medical record, or death from any cause during the study timeframe. Response rates were defined by Response Evaluation Criteria in Solid Tumors (RECIST version 1.1) [11]. PFS was censored at the last date of follow-up or at the last date of mFOLFOX6 administration if a patient was lost to follow-up, received palliative surgery, or changed therapy because of inconvenience or intolerability. Secondary endpoints included OS, which was defined as the time from the date of mFOLFOX6 therapy initiation until the date of death from any cause. If a patient did not progress or expire, PFS and OS were censored at the data analysis cutoff date. Additionally, the secondary endpoint of DCR was defined as complete response, partial response, or stable disease at first scan by means of an imaging study (defined by RECIST version 1.1) [11]. If imaging was not available, DCR was defined on the basis of documentation in clinical notes. Safety endpoints included the use of growth factor support and the incidence of adverse events (AEs). The severity of AEs was graded in accordance with the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 [12]. These events were documented if they occurred at least once at any time during treatment. When possible, the specific dosing observations of the regimen were recorded. The version of FOLFOX used at MCC is mFOLFOX6, which consists of oxaliplatin 85mg/m2 IV and LV 400mg/m2 IV administered over 2 hours on day 1. This is followed by 5-FU 400 mg/m2 IV bolus administered over 5 minutes and 5-FU 2400mg/m2 IV administered as a continuous infusion over 46 hours. Each cycle is repeated every 2 weeks [13].
Statistical Analysis
Baseline patient and disease characteristics were summarized using descriptive statistics. Kaplan-Meier curves were used to estimate median PFS and OS with 95% confidence intervals. Multivariable cox proportional-hazards model was used to provide estimated hazard ratio (HR) and confidence intervals for PFS and OS. Sample size was powered based on 200 patients. On the basis of historical data showing an overall median PFS of 10 months, we expected 25% of patients to have received the bolus (n = 50) [14]. The given sample size was powered at 87% to detect a 25% difference of progression rate between the bolus and non-bolus groups based on a one-sided log-rank test, with a 5% type I error.
Results
Patients and treatment
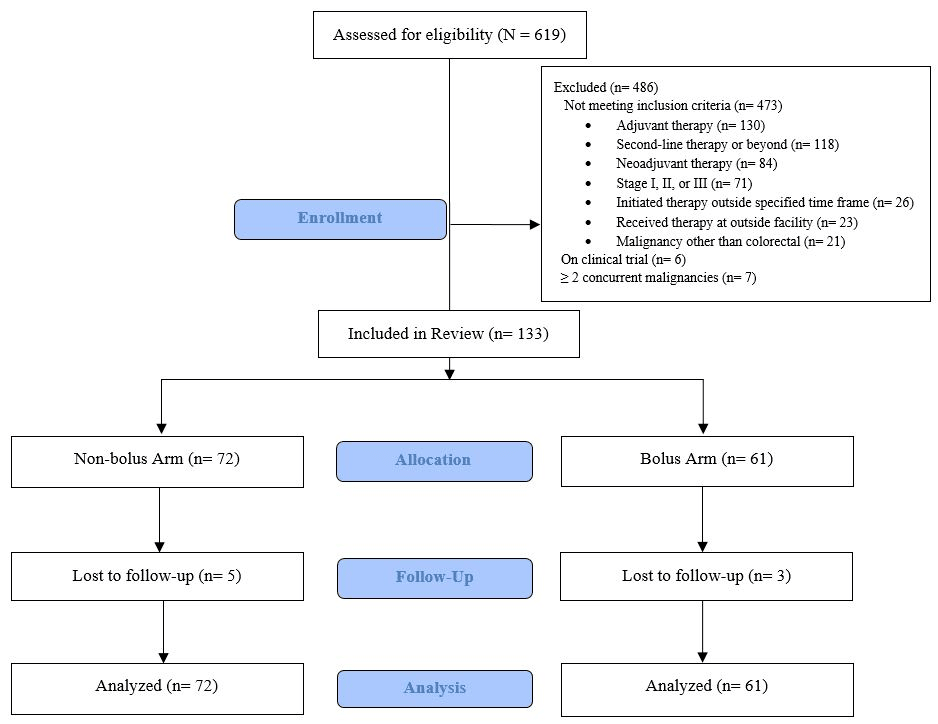
Figure 1:Consort Diagram
Out of 619 patients who were screened, a total of 133 met the eligibility criteria. The key reasons for exclusion included receiving mFOLFOX6 in the non-palliative setting (as adjuvant or neoadjuvant therapy) and as a subsequent line of therapy. Seventy-two patients were included in the non-bolus arm and 61 in the bolus arm (Figure 1). Overall, baseline patient or disease characteristics were balanced between the groups, with the exception of their sites of metastatic disease (Table 1). There were fewer patients with liver metastases in the non-bolus arm than in the bolus arm (38% vs 57%; P = 0.025, respectively). In the non-bolus arm, 44% of patients had multiple sites of metastatic disease versus only 11% of patients in the bolus arm (P = 0.001) (Table 1).
At the time of data cutoff (December 31, 2019), median follow-up was 21.8 months for PFS. Median treatment duration in the non-bolus arm was 5.5 months and 4.8 months in the bolus arm. Incidence of cycles delayed at any time was observed in 56% of non-bolus and 61% of bolus patients. Bevacizumab therapy was used in combination with the mFOLFOX6 regimen for 83% of all study patients. Table 2 describes dosing and regimen observations between the 2 groups. These events were documented if they occurred at least once at any time during treatment. The non-bolus arm had a statistically higher total amount of completed chemotherapy cycles, with a median of 12.5 cycles versus 10 cycles in the bolus arm (P = 0.045). The median number of completed cycles with oxaliplatin was similar in both arms: 8 cycles in the bolus arm vs 9 cycles in the non-bolus arm (P = 0.079).
Efficacy
Table 1:Baseline Patient and Disease Characteristics.
| Characteristics | Non-bolus (n=72) | Bolus (n=61) | P value |
|---|---|---|---|
| Age in years, median (range) | 61 (27-86) | 64 (26-89) | 0.307 |
| Male, n (%) | 38 (53) | 32 (53) | 1.000 |
| ECOG performance status, n (%) | |||
| 0 | 30(42) | 30(49) | 0.484 |
| 1 | 38(52) | 26(43) | 0.297 |
| 2 | 4(6) | 5(8) | 0.732 |
| Location of primary tumor, n (%) | |||
| Right | 22(31) | 11(18) | 0.284 |
| Left | 33(46) | 31(51) | 0.604 |
| Rectal | 8(11) | 12(20) | 0.224 |
| Rectal/Left | 9(12) | 7(11) | 1.000 |
| Sites of metastatic disease, n (%) | |||
| Liver | 27(38) | 35(57) | 0.025 |
| Lung | 3(4) | 6(10) | 0.300 |
| Multiple | 32(44) | 7(11) | 0.001 |
| Other* | 10(14) | 13(21) | 0.358 |
| Molecular status, n (%) | |||
| MSS | 66(97) | 53(95) | 0.657 |
| BRAFWT | 64(94) | 50(93) | 0.731 |
| RASWT | 37(54) | 36(68) | 0.137 |
| Previous exposure to chemotherapy, n (%) | |||
| 5-FU based therapy | 2 (3) | 4 (6) | 0.412 |
| Oxaliplatin-based therapy | 1 (1) | 3 (5) | 0.332 |
| None | 9 (96) | 54 (89) | 0.185 |
*Other sites include lymph nodes, bones, or visceral organ involvement other than liver or lung. Abbreviations: ECOG, Eastern Cooperative Oncology Group; MSS, Microsatellite Stable; WT, wild type
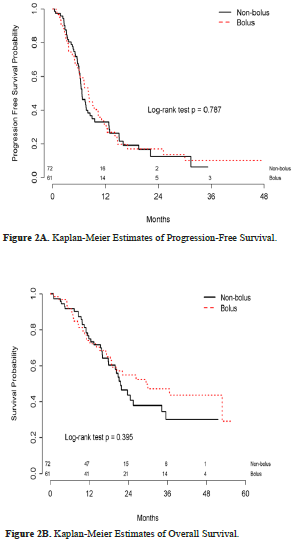
No statistical difference was found in the primary endpoint of median PFS between the bolus (8.12 months) and non-bolus arms (6.64 months) (HR, 0.083; 95% CI, 0.51-1.36; P = 0.470) (Figure 2A). At the time of our analysis, 66 deaths had occurred: 36 in the non-bolus arm and 30 in the bolus arm. No statistical difference was observed in median OS between the bolus (29.4 months) and non-bolus arms (21.6 months) (HR, 0.62; 95% CI, 0.37-1.05; P = 0.395) (Figure 2B).
Responses at first scan are shown in Table 3. DCR at first scan occurred in 84% of study patients, which was similar between the bolus and non-bolus arms (86% vs 82%; P = 0.44, respectively). Of note, 3 patients in the bolus arm did not undergo imaging. Therefore, response at first scan could not be assessed. There were no significant differences in the other measures of response at first scan, including partial response, stable disease, and progressive disease, between the 2 groups. There were no complete responses in either group.
Safety
At least 1 hematologic AE (any grade) was observed in 60 (98%) patients in the bolus arm and 69 (96%) patients in the non-bolus arm (P = 0.625). Grade 3 or higher treatment-related hematologic AEs were more frequently observed in the bolus arm (38%) than in the non-bolus arm (22%) (P = 0.058). There were no significant differences in peripheral neuropathy or mucositis between the non-bolus and bolus arms (Table 3). Additionally, growth factor support was used for 23% of patients in the bolus arm versus 7% in the non-bolus arm (P = 0.012) (Table 2).
Table 2:Dosing and Regimen Observations*.
| Non-bolus (n=72) | Bolus (n=61) | P value | |
|---|---|---|---|
| Treatment regimen, n (%) | |||
| mFOLFOX6 + bevacizumab | 61 (85) | 49 (80) | 0.646 |
| mFOLFOX6 + cetuximab | 1 (1) | 1 (2) | 1.000 |
| mFOLFOX6 | 10 (14) | 11 (18) | 0.634 |
| Total treatment duration (months), median (range) | 5.5 (0-31.5) | 4.8 (0-46.8) | 0.224 |
| Total mFOLFOX6 cycles completed, median (range) | 12.5 (1-70) | 10 (1-80) | 0.045 |
| With oxaliplatin | 9 (1-22) | 8 (1-14) | 0.079 |
| Cycles delayed at any time, n (%) | 40 (56) | 37 (61) | 0.600 |
| Time (months) to oxaliplatin discontinuation, median (range) | 4.36 (0.7-38.8) | 4.37 (0.5-15.4) | 0.413 |
| 5-FU CIVI reductions, n (%) | 11 (15) | 14 (24) | 0.266 |
| Oxaliplatin reductions, n (%) | 27 (38) | 23 (39) | 1.000 |
| Utilization of growth factor support, n (%) | 5 (7) | 14 (23) | 0.012 |
| Cycle number growth factor added, mean (SD) | 0.46 (1.3) | 0.28 (1.15) | 0.014 |
*Results for modifications to the chemotherapy regimen are reported as at least 1 event that occurs while receiving treatment. Abbreviations: mFOLFOX6, 5-FU + LV + Oxaliplatin; SD, standard deviation
Discussion
In this retrospective review, we found no significant differences in PFS and OS when 5-FU bolus was empirically eliminated from mFOLFOX6 therapy. In terms of safety, patients who received 5-FU bolus experienced a higher incidence of hematologic AEs. Patients receiving 5-FU bolus also required an increased use of granulocyte-colony stimulating factor.
The Meta-Analysis Group in Cancer study compared 5-FU bolus with 5-FU continuous infusion in the treatment of patients with colon cancer [9,10]. This study found that 5-FU bolus was associated with increased hematologic AEs compared with 5-FU infusion. AE findings in our study were similar to those of the Meta-Analysis Group in Cancer study. However, the Meta-Analysis Group in Cancer study was conducted before FDA approval of oxaliplatin. Therefore, the FOLFOX regimen was not established as the standard of care at that time, and data for the removal of bolus are lacking with this regimen. Ours is one of the few studies to date to analyze the safety and efficacy impacts of empirically eliminating 5-FU bolus from first-line mFOLFOX.
Table 3:Response at First Scan and Adverse Events
| Non-bolus (n=72) | Bolus (n=58) | P value | |
|---|---|---|---|
| Response, n (%) | |||
| DCR | 59 (82) | 53 (86) | 0.440 |
| Partial responses | 26 (36) | 23 (39) | 0.718 |
| Stable disease | 33 (46) | 27 (47) | 1.000 |
| Progressive disease | 13 (18) | 8 (14) | 0.633 |
| Any Grade, n (%) | |||
| Neutropenia | 32 (44) | 39 (64) | 0.036 |
| Anemia | 66 (92) | 55 (90) | 0.772 |
| Thrombocytopenia | 41 (57) | 34 (56) | 1.000 |
| Any | 69 (96) | 60 (98) | 0.625 |
| Grade 3/4, n (%) | |||
| Neutropenia | 9 (13) | 12 (20) | 0.341 |
| Anemia | 8 (11) | 12 (20) | 0.224 |
| Thrombocytopenia | 2 (3) | 3 (5) | 0.660 |
| Any | 16 (22) | 23 (38) | 0.058 |
| Any Grade, n (%) | |||
| Mucositis | 6 (8) | 10 (16) | 0.186 |
| HFS | 1 (1) | 1 (1) | 1.000 |
| Peripheral neuropathy | 46 (64) | 36 (59) | 0.595 |
| Grade 3/4, n (%) | |||
| Mucositis | 1 (1) | 1 (1) | 1.000 |
| HFS | 0 (0) | 0 (0) | 1.000 |
| Peripheral neuropathy | 0 (0) | 1 (1) | 0.459 |
Abbreviations: HFS, hand-foot syndrome.
Data from this retrospective study was in keeping with previous randomized trials where FOLFOX was used with or without bevacizumab, achieving an estimated PFS of 8 months [14].
Although there were no statistically significant differences in OS between the 2 groups, the bolus arm had a numerically higher median OS rate than the non-bolus arm at 29.4 and 21.6 months, respectively. There may be a few explanations for this difference. Firstly, our study did not reach power for the primary endpoint of PFS. Additionally, at baseline, only 11% of patients in the bolus arm had multiple metastatic sites compared to 44% of patients in the non-bolus arm. This difference might represent an inherent bias of the treating provider to opt for a less aggressive option by empirically eliminating 5-FU bolus when treating patients with widespread metastatic disease. Lastly, in the Kaplan-Meier curves (Figures 2A and 2B), the separation of curves starts at approximately 24 months. At this time point, less than one-third of patients remained in each arm, further reducing statistical significance. The bolus arm used more growth factor support and had more hematologic AEs. These findings emphasize the importance of avoiding unwanted AEs, especially when using a treatment regimen that is used in the palliative setting.
We do recognize the potential for biases due to the retrospective nature of the study. Although the patient population was balanced between the two arms, one could argue that patient-driven factors such as performance status and overall clinical condition could impact an individual provider’s decision on eliminating 5-FU/LV irrespective of their practice pattern. In addition, the relatively small size cohort may not provide adequate granularity when other prognostic factors such as molecular profiling and tumor sidedness are to be considered.
In conclusion, this retrospective analysis found that empiric elimination of 5-FU bolus and LV when treating mCRC patients with mFOLFOX6 had no statistically significant impact on PFS, OS, and DCR. Removal of 5-FU bolus and LV was associated with a favorable safety profile and reduced utilization of growth factor support, which would be a desirable outcome in the palliative setting. Future prospective studies are needed to better inform treatment decisions to use or remove 5-FU bolus in treating patients with mCRC receiving palliative therapy.
Acknowledgement of Research support
The authors wish to acknowledge Jennifer Swank, Mark Vragovic, and the study and biostatistics team, including Dung-Tsa Chen and Jun-min Zhou. Editorial assistance was provided by the Moffitt Cancer Center’s Scientific Editing Department by Dr. Paul Fletcher and Daley Drucker. No compensation was given beyond their regular salaries.
Disclaimers
The authors have no conflicts of interest to declare. This research was published as an abstract and presented as a poster presentation at the 2020 ASCO Annual Meeting on May 29, 2020.
References
1. American Cancer Society. 2020. Cancer Facts and Figures.
2. National Comprehensive Cancer Network. (2020). Colon Cancer (version 4.2020).
3. Colucci G, Gebbia V, Paoletti G, et al. Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the Gruppo Oncologico Dell’Italia Meridionale. J Clin Oncol. 2005; 23:4866-4875.
4. de Gramont Ad, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000; 18:2938-2947.
5. Tournigand C, André T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229-237.
6. Aschele C, Sobrero A, Faderan MA, et al. Novel mechanism (s) of resistance to 5-fluorouracil in human colon cancer (HCT-8) sublines following exposure to two different clinically relevant dose schedules. Cancer res. 1992; 52:1855-1864.
7. Sobrero AF, Aschele C, Bertino JR. Fluorouracil in colorectal cancer--a tale of two drugs: implications for biochemical modulation. J Clin Oncol. 1997; 15:368-381.
8. Fraile RJ, Baker LH, Buroker TR, et al.Pharmacokinetics of 5-fluorouracil administered orally, by rapid intravenous and by slow infusion. Cancer Res. 1980; 40:2223-2228.
9. Meta-Analysis Group in Cancer. Efficacy of intravenous continuous infusion of fluorouracil compared with bolus administration in advanced colorectal cancer. J Clin Oncol. 1998;16(1):301-8.
10. Meta-Analysis Group in Cancer. Toxicity of fluorouracil in patients with advanced colorectal cancer: effect of administration schedule and prognostic factors. J Clin Oncol. 1998;16(11):3537-41.
11. Eisenhauer EA, Therasse P, Bogaerts J, et al: New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). European journal of cancer 45:228-247, 2009.
12. National Cancer Institute, National Institutes of Health, US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE), Version 5.0. Published November 27, 2017; Revised December 17, 2019. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_5.0/. Accessed December 1, 2019.
13. Grothey A, Sargent D, Goldberg RM, et al. Survival of patients with advanced colorectal cancer improves with the availability of fluorouracil-leucovorin, irinotecan, and oxaliplatin in the course of treatment. J Clin Oncol. 2004; 22:1209-1214.
14. Saltz LB, Clarke S, Díaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008; 26:2013-2019.
Received: April 26, 2021;
Accepted: May 19,2021;
Published: May 31, 2021.
To cite this article : Alexa J. Basilio, Anand B. Shah, Katelyn R. Sommerer, et al. Impact of Empirically Eliminating 5-Fluorouracil Bolus and Leucovorin in Patients with Metastatic Colorectal Cancer Receiving First-Line Treatment with mFOLFOX6. British Journal of Cancer Research. 2021; 4:1.
©2021 Alexa J. Basilio, et al.
