Case Report / Open Access
DOI: 10.31488/bjcr.167
Long-term, 25-year Survival Following Surgery and Immune Cell Therapy Combined with Chemotherapy for HER2-Positive Metastatic Breast Cancer:
Goki Shindo*1, Takayoshi Endo1, Yoju Miyamoto2, Masamitsu Onda1, Masayuki Kimura3, Rishu Takimoto4,5, Hiroshi Ibe4, Shigenori Goto4,5
1. Meditopia Numazu Clinic, 575-1 Okaisshiki, Numazu City, Shizuoka Prefecture 410-0012, Japan
2. Shin-Yamate Hospital, 3-6-1 Suwamachi, Higashi- Murayama City, Tokyo 89-0012, Japan
3. Department of Surgery, Numazu City Hospital, 550 Harunoki, Higashi-Shiiji, Numazu City, Shizuoka Prefecture 410-0302, Japan
4. Seta Clinic Group, New Surugadai Bldg. 3F, 2-1-45 Kandasurugadai, Chiyoda-ku, Tokyo 101-0062, Japan
5. Department of Next Generation Cell and Immune Therapy, Graduate School of Medicine, Juntendo University, 2-1-1 Hongo, Bunkyo City, Tokyo 113-8421, Japan
*Corresponding author: Goki Shindo, MD, Director, Meditopia Numazu Clinic, 575-1 Okaisshiki, Numazu City, Shizuoka Prefecture 410-0012, Japan
Abstract
The surgical cure rate for breast cancer is relatively high among the various cancers thanks to recent advances in perioperative hormonal therapy and chemotherapy. However, the 10-year survival rate for stage 4 breast cancer is only 16% in Japan. Here we report a case of 25-year survival following primary right radical mastectomy with axillary lymph node dissection at age 46 years (in 1996) and subsequent immunotherapy and chemotherapy. Pathological diagnosis was stage 3A (T3aN2M0), and histochemical diagnosis was estrogen receptor(−), progesterone receptor(+), HER2(+). During postoperative year 1 (POY1), chemotherapy and focal radiation therapy were added for a solitary metastatic lesion to a right cervical lymph node. Continuous chemotherapy for HER2(+) metastatic breast cancer(MBC)was performed for 8 years. In POY8, side effects of chemotherapy became intolerable, tumor marker levels increased, and lymphodepletion developed. Following immune cell therapy with activated T lymphocytes (ATL)she recovered completely with no adverse events for the next 10 years. In POY18, a second, massive administration of ATL was required because of right-sided malignant pleural effusion. She recovered again to ECOG performance status(PS) 0 at 1 year after the immunotherapy combined with chemotherapy. Twenty-five years after initial surgery, the patient continues to be well. We discuss several important factors for predicting the effectiveness of immune cell therapy combined with chemotherapy during long-term follow-up. The most important are lymphodepletion and trends in the CD4/CD8 ratio, and other considerations are the effectiveness of the chemotherapeutic agents combined with immune cell therapy, tumor markers, and ECOG PS.
Keywords: Metastatic breast cancer, long-term survivorship, immune cell therapy, chemotherapy, CD4/CD8 ratio
Introduction
Breast cancer is among the most common solid cancers with a favorable 10-year survival rate after radical surgery [1]. In particular, remarkable progress in pre- and postoperative chemotherapy and hormonal therapy has contributed to improved survival in patients with breast cancer [2, 3]. Among Japanese women, breast cancer is the leading cause of cancer morbidity, with 92,605 cases in 2019, but only the fifth leading cause of cancer mortality, with 14,839 deaths in 2019. However, prognosis remains poor in some groups of patients. About 25% of women with breast cancer have metastatic disease, and the 10-year survival rate following surgery for stage 4 disease was only 16% according to a 2021 survey in Japan [1].
Immune cell therapy using ATL(αβΤ cell therapy) has several effects incompatible with chemotherapy such as induction of lymphopenia and immunosuppression, especially in high-dose regimens [4]. On the other hand, synergistic effects of these two types of therapy include induction of tumor cell death, elimination of regulatory T cells, enhancement of tumor cell sensitivity to lysis by cytotoxic T lymphocytes [5], and increased efficacy of adoptive lymphocyte transfer. Immunotherapy may directly modulate the sensitivity of tumor cells to chemotherapy. Furthermore, targeted immunotherapy with a checkpoint inhibitor in combination with chemotherapy has become actively used as adjuvant therapy before and after radical surgery for triple-negative breast cancer(TNBC) [6] and metastatic metaplastic breast cancer(MMBC) [7].
Case Presentation
The patient was a 46-year-old woman (height 155 cm, weight 49 kg) who underwent radical mastectomy with right axillary lymph node dissection for an 11-cm tumor of the right breast in 1996. Pathological diagnosis showed an 11-cm-long, 3-cm-thick main tumor of stage 3A (T3aN2M0), and the immunohistochemical diagnosis was estrogen receptor(−), progesterone receptor(+), HER2(+).
A standard postoperative chemotherapy regimen for HER2(+) MBC (methotrexate 40 mg/kg, cyclophosphamide100 mg/kg, and 5-fluorouracil (5-FU) 500 mg/kg intravenous injection) was administered 10 times over the first 5 years. During this period, additional skin excisions were performed on 8 occasions for recurrent rice grain-sized skin metastases near the skin incision for the primary surgery. For additional postoperative chemotherapy, doxorubicin hydrochloride 20 mg/kg was administered 9 times over the course of 1 year in POY4. Focal radiation therapy was added for a solitary metastatic lesion to a right cervical lymph node and it disappeared completely. Additional chemotherapy was added periodically using paclitaxel 60 mg/m2 every 2 weeks with a 1-week break and trastuzumab 6 mg/kg intravenously every 3 weeks without severe side effects.
Right-sided cervical lymph node swelling appeared again in POY8 and high-dose chemotherapy with paclitaxel 90 mg/week severely impaired the patient’s physical condition. Even after a dose reduction to 60 mg/week, the side effects of nausea, vomiting, abdominal pain, and diarrhea were intolerable, her tumor marker levels were increased, and she developed severe lymphodepletion (lymphocyte count <620/μl) , so she visited our clinic seeking more tolerable postoperative cancer therapy. We administered 2 courses of immune cell therapy using αβT cells (Figure 1a). The αβT cells were generated as described previously [8, 9]. Briefly, peripheral blood mononuclear cells (PBMCs) were isolated from the patient’s peripheral blood using a Vacutainer (Becton, Dickinson and Company, Franklin Lakes, NJ). The PBMCs were activated in a culture flask with an immobilized monoclonal antibody to CD3 (Jansen-Kyowa, Tokyo, Japan) in Hymedium 930 (Kohjin Bio, Saitama, Japan) containing 1% autologous serum. The PBMCs were then cultured for 14 days with 700 IU/ml recombinant interleukin 2 (IL-2);Proleukin®; Chiron, Amsterdam, the Netherlands), after which, (3–10) × 109 cells were harvested and suspended in 100 ml of normal saline for intravenous injection. αβΤ cell therapy is commonly administered 6 times (every 2 weeks for 3 months) in 1 course.
Following treatment, she recovered from the severe lymphodepletion, tumor markers returned to normal levels, and her ECOG PS improved to 0, indicating a return to her previous level of daily activities. For the next 10 years, she received additional αβT cell therapy intermittently with 1 intravenous administration every 3 to 6 months, for a total of 34 times up to POY18 (2014). This immune cell therapy was combined with additional postoperative chemotherapy using trastuzumab and anastrozole (Figure 1a) without adverse effects.
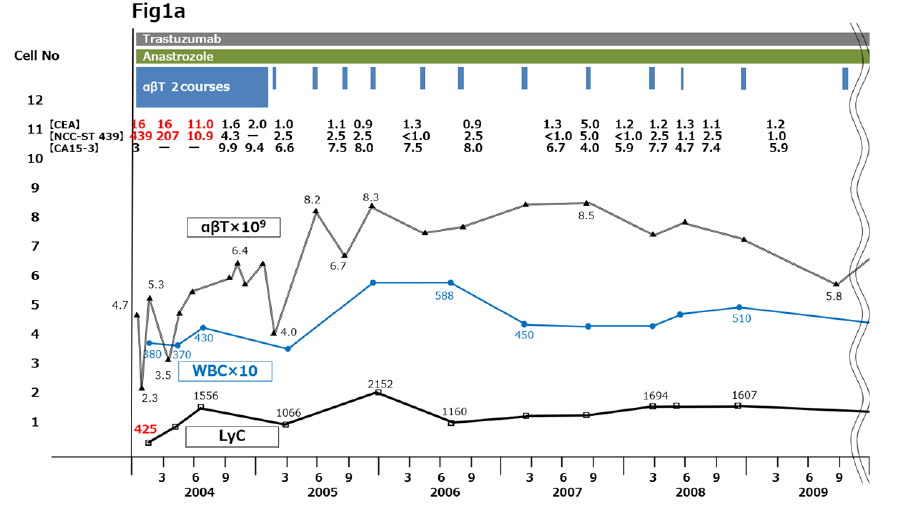
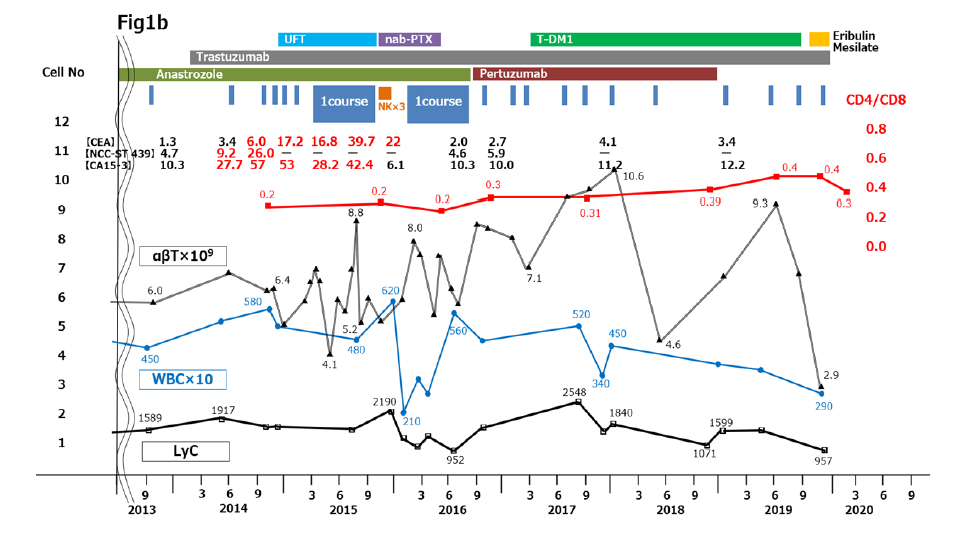
Figure 1:Clinical course from 2004 to 2020. (a) 2004 to 2011. (b) 2012 to 2020. Immune cell therapy and chemotherapy are shown in the upper part of the panels. Changes in tumor marker levels are shown as tabulated numerical values. Plotted graphs show changes in αβΤ cell counts (thin black line), WBC counts (blue line), LyC (thick black line), and CD4/CD8 ratio (red line). CA15-3, cancer antigen 15-3; CEA, carcinoembryonic antigen; LyC, lymphocyte count; WBC, white blood cell count
In POY18, the patient felt physical dullness, became easily fatigued on movement, and had shortness of breath despite continuous chemotherapy with oral anastrozole 1 mg/day. She revisited Numazu City Hospital, where she had undergone primary surgery, and pleural effusion in the right thorax was found on chest computed tomography (CT) (Figures 1b and 2A). Breast cancer cells were found in the pleural fluid, and stage 4 MBC with dissemination to the right thoracic cavity was diagnosed.
Two courses of immune cell therapy with administration of massive amounts of αβT cells were carried out, along with 3 intravenous injections of activated NK cells, which we prepared by ex vivo culture with IL-2 and other cytokines. Additional intermittent αβT cell injections once every 1 or 2 months were continued in combination with chemotherapy for 1 year. The malignant pleural effusion completely disappeared on CT 1 year later (Figure 2B) and tumor markers returned to normal levels. The CD4/CD8 ratio was almost unchanged when measured before and after one or 2 courses of immune cell therapy meaning that she remained immunocompetent during this period (Figure 1b) [10].
Discussion
As of 2021, 25 years after the primary radical surgery, the patient is now 71 years old and continues to be well, with no adverse events. To our knowledge, this is the longest surviving case of MBC treated with immune cell therapy using ATL and combination chemotherapy that has been reported in the literature.
Survival outcomes following surgery for breast cancer treated with current hormonal therapy or chemotherapy are some of the best among solid cancers. However, because of relapse or metastasis, stage 4 breast cancer still has a poor 10-year survival rate of 16%, which is similar to that of esophageal cancer (14.2%) and colon cancer (11.1%) [1]. Conventional treatments including surgery, chemotherapy, and radiotherapy can have various adverse effects and impair antitumor immunity, resulting in residual disease. Since the early 1990s in Japan, αβT cell therapy has been shown to be a promising and effective treatment, with almost no adverse effects, compared with conventional chemotherapies for postoperative cancer patients [11].
A clinical study conducted in 2015 revealed that in patients with HER2(+) metastatic breast cancer, survival was significantly better with pertuzumab plus trastuzumab than with docetaxel alone [3], which had been regarded as an effective first-line treatment for MBC [12]. Moreover, for TNBC and chemo-refractory MMBC, checkpoint inhibitor therapy combined with chemotherapy has emerged as a new clinical paradigm [6, 7]. Immune cell therapy using ATL has been demonstrated to reduce the activity of regulatory T cells and to provide a sufficient supply of activated T lymphocytes (CD8+T cells) [13].
In comparisons between ATL therapy alone and in combination with chemotherapy, several studies have demonstrated that combination therapy achieves a better therapeutic effect for HER2(+) MBC [4, 14]. Capecitabine, a prodrug of 5-FU, upregulates tumor antigen expression and selectively kills tumor-associated myeloid-derived suppressor cells, resulting in increased T cell-dependent antitumor immunity [15]. These findings suggest that the chemotherapeutic agents used in our case (5-FU, tegafur/uracil, taxanes, anthracyclines) can have several beneficial effects on the immune system and antitumor immunity specifically, resulting in improved prognosis. However, recovery from lymphodepletion in our patient seems to have been achieved by supplying CD8(+)T lymphocytes via multiple infusions of ATL in immune cell therapy. It is worthwhile to know the total number of αβΤ cells administered to patients in order to determine the increases in activated CD8(+) T lymphocytes (Figure 1a, b), [10, 13]. Recovery and maintenance of immunocompetence can be assessed based on changes in the CD4/CD8 ratio during the follow-up period. A downward curve or plateau of the CD4/CD8 ratio indicates immunocompetence, whereas an upward curve of the CD4/CD8 ratio indicates immunodeficiency [10]. The pattern of changes in the CD4/CD8 ratio is a useful clinical marker to understanding immunological status in cancer patients during follow-up and is cost-effective for frequent measurements.
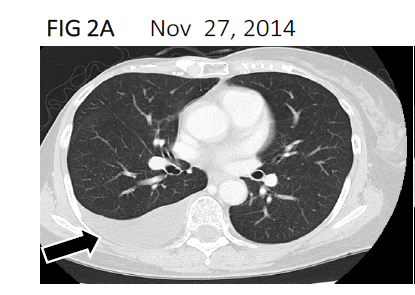
Figure 2A:Computed tomography scan showing pleural effusion(black arrow) in the right thorax cavity in November 2014
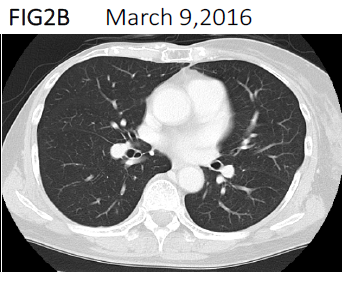
Figure 2B:Computed tomography scan showing complete disappearance of the pleural effusion in March. 2016
Conclusions
We have described a case of long-term, 25-year survival in a patient with MBC who was treated with postoperative immune cell therapy using ATL combined with chemotherapy. Judging from this case, the most important factors for estimations of postoperative long survival are to find lymphodepletion as soon as possible and trends in the CD4/CD8 ratio to estimate patient’s active immune ability, and other considerations are to find the effectiveness of the chemotherapeutic agents combined with immune cell therapy, tumor markers and ECOG PS.
Immune cell therapy with chemotherapy may be an effective option for MBC patients following surgery. Immune cell therapy administered with appropriate timing can resolve lymphodepletion induced by chemotherapy and the pattern of change in the CD4/CD8 ratio can provide useful information about the patient’s immunological status. Together with conventional tumor markers and performance status, the CD4/CD8 ratio can be useful for predicting prognosis in the follow-up of cancer patients.
This case demonstrates the effectiveness of immunotherapy for metastatic HER2(+) breast cancer and highlights the potential of the emerging paradigm of combining immune checkpoint inhibitors with chemotherapy in cancer therapy.
Abbreviations
αβΤ cell therapy: immune cell therapy using activated T lymphocytes; ATL: activated T lymphocytes; CT: computed tomography; DC: dendritic cell; IL: interleukin; MBC: metastatic breast cancer; MMBC: metastatic metaplastic breast cancer; PS: performance status; TNBC: triple-negative breast cancer
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflicts of Interest
The authors declare that there are no potential conflicts of interest.
Availability of Data and Material
All data generated or analyzed during this study are included in the published article.
Code Availability
Not applicable.
Authors’ Contributions
Conception and design: G. Shindo, T. Endo, and M. Kimura. Data collection and assembly of data: Y. Miyamoto, M. Onda, M. Kimura, H. Ibe, R. Takimoto, and S. Goto. Analysis and interpretation: G. Shindo and T. Endo. Final approval of manuscript: All authors.
Ethical Approval
The patient was treated according to the study protocol of a clinical trial registered at Japan Registry of Clinical Trials (jRCTc030190255) and approved by the Research Ethics Committee of Seta Clinic (approval number: SCG68).
Consent for Publication
The patient has consented to the submission of the case report to the journal.
References
1. National Cancer Center Japan. Latest report of cancer statistics of Japan. 2019. https://ganjoho.jp/reg_stat/statistics/brochure/hosp_c_reg_surv.html.
2. Harbek N, Jakesz R. St. Gallen 2007: Breast cancer treatment consensus report. Breast Care. 2007; 2:130-134. https://doi.org/10.1159/000103629
3. Swain SM, Baselga J, Kim S-B, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015; 372:724-734. https://doi.org/10.1056/NEJMoa1413513
4. Zhang T, Herlyn D. Combination of specific immunotherapy or adoptive antibody or lymphocyte immunotherapy with chemotherapy in the treatment of cancer. Cancer Immunol Immunother. 2009; 58:476-492. https://doi.org/10.1007/s00262-008-0598-y
5. Zhang B, Bowerman NA, Salama JK, et al. Induced sensitization of tumor stroma leads to eradication of established cancer by T cells. J Expel Med. 2007; 204:49-55. https://doi.org/10.1084/jem.20062056
6. Cyprian FS, Akhtar S, Gatalica Z, et al. Targeted immunotherapy with a checkpoint inhibitor in combination with chemotherapy: a new clinical paradigm in the treatment of triple–negative breast cancer. Bosn J Basic Med Sci. 2019; 19:227-233. https://doi.org/10.17305/bjbms.2019.4204
7. Al Sayed AD, Elshenawy MA, Tulbah A, et al. Complete response of chemo-refractory metastatic metaplastic breast cancer to paclitaxel-immunotherapy combination. Am J Case Rep. 2019; 20:1630-1635. https://doi.org/10.12659/AJCR.918770
8. Egawa K. On the safety assurance of all processing carried out on medical institutions for autologous immune-cell therapy. Hum Cell. 2004; 17:1-6. http://dx.doi.org/10.1111/j.1749-0774.2004.tb00014.x
9. Takimoto R, Kamigaki T, Okada S, et al. Efficacy of adoptive immune-cell therapy in patients with advanced gastric cancer: A retrospective study. Anticancer Res. 2017; 37:3947-3954. https://doi.org/10.21873/anticanres.11778
10. Shindo G, Endo T, Onda M, et al. Is the CD4/CD ratio an effective indicator for clinical estimation of adoptive immunotherapy for cancer treatment? J Cancer Ther. 2013; 4:1382-1390. https://doi.org/10.4236/jct.2013.48164
11. Egawa K. Immuno-cell therapy in Japan. Anticancer Res. 2004; 24:3321-3326.
12. Gennari A, Conte P, Rosso R, et al. Survival of metastatic breast carcinoma patients over a 20-year period. A retrospective analysis based on individual patient data from six consecutive studies. Cancer. 2005; 104:1742-1750. https://doi.org/10.1002/cncr.21359
13. Noguchi A, Kaneko T, Naitoh K, et al. Impaired and imbalanced cellular immunological status assessed in advanced cancer patients and restoration of the T cell immune status by adoptive T-cell immunotherapy. Int Immunopharmacol. 2014; 18:90-97. https://doi.org/10.1016/j.intimp.2013.11.009
14. Costa RLB, Czerniecki BJ. Clinical development of immunotherapies for HER2+ breast cancer: A review of HER2-directed monoclonal antibodies. NPJ Breast Cancer. 2020; 6:1-45. https://doi.org/10.1038/s41523-020-0153-3
15. Heinhuis KM, Ros W, Kok M, et al. Enhancing antitumor response by combining immune checkpoint inhibitors with chemotherapy in solid tumors. Ann Oncol. 2019; 30(2)219-235. https://doi.org/10.1093/annonc/mdy551
Received: August 04, 2021;
Accepted: August 23, 2021;
Published: August 26, 2021.
To cite this article : Shindo G, Endo T, Miyamoto Y, et al. Long-term, 25-year Survival Following Surgery and Immune Cell Therapy Combined with Chemotherapy for HER2-Positive Metastatic Breast Cancer: A Case Report. British Journal of Cancer Research. 2021; 4:2.
©2021 Shindo G, et al.
