Case report/ Open Access
DOI: 10.31488/bjcr.164
Non Hodgkin’s Lymphoma T cell (PTCL-NOS) Post Salvage Radiation, a Long Term Survivor
Jovita Martin Daniel*1, Anita Ramesh2, Kalaichelvi K3
1.Associate Professor Medical Oncology Sri Ramachandra Institute of Higher Education and Research
2. Professor Medical Oncology Saveetha Medical College
3. Professor Medical Oncology Consultant Apollo Hospital
*Corresponding author:Dr. Jovita Martin Daniel, Associate Professor Medical Oncology Sri Ramachandra Institute of Higher Education and Research, India
Abstract
A 47 year old Premenopausal lady presented NHL T cell lymphoma failed 2 lines of Chemotherapy. She was given stem cell transplant as the next resort. Being in a resource restricted setting, she was given radiation [total nodal radiation with mantle field and inverted Y treated sequentially] as a salvage treatment. After which, the patient has been on follow up for 10 years with her serial PET CT scans showing complete remission. This case is discussed highlighting the importance of salvage radiation in Lymphomas in the relapsed setting which is often under used and undermined.
Key words: Salvage radiation, PTCL, Long term Survivor NHL
Background
In PTCL the outcomes of patients are generally poor; there are no well established care standards for relapsed/refractory PTCL. There are several therapeutic options [1]. Guidelines on the management for PTCL included stem cell transplantation as one therapeutic option [2, 3]. However, resource constrained setting happens to be the most important limiting reason for salvage treatments. This is such a scenario where this patient was left with no financial assistance for further immunotherapy or stem cell transplant. Hence as a last resort was given salvage radiation.
Case Presentation
A 47 year old Premenopausal lady presented with a swelling sub mental region in 2010, she developed intermittent fevers, night sweats, and fatigue. Initial physical exam revealed multiple cervical, axillary and inguinal lymph nodes and routine blood work were both unremarkable. Hypothyroidism was the only co morbidity, she had for 5 years prior to the diagnosis of PTCL-NOs. There was an initial diagnostic dilemma of whether it was Hodgkin’s lymphoma classical type versus NHL T cell type with the excision biopsy from the neck lymph node. A repeat cervical whole lymph node excision biopsy from the lesion however confirmed Peripheral T cell lymphoma. The Immunohistochemistry was positive for LCA, CD3 ,CD 5,CD 30, CD45, PAX 5; negative for CD20,CD21; Ki67 (30 to 40%). She underwent standard staging studies including computer tomography of the chest, abdomen, and pelvis. Pre Chemo CT scan results shows bilateral cervical lymph node, 2 x 1cm each, sub mental LN 2 x 1 cm, bilateral inguinal lymph node present. Bone marrow aspiration was normal. Chest x-ray and USG Abdomen were normal. Diagnosis of T cell NHL Stage IVB was established, she was treated with 8 cycles CHOP regimen [Day 1: Cyclophosphamide 750mg/m2 IV + doxorubicin 50mg/m2 IV bolus + vincristine 1.4mg/m2 IV bolus (max dose 2mg), Prednisone 100mg orally 5 days) from June 2009 to November 2009. She relapsed in 6 months with right upper cervical lymph node, Left supra clavicular lymph node. The biopsy of neck lymph nodes showed PTCL-NOS. Hence, she was given 6 cycles of ICE regimen [Ifosfamide: 5 g/m2, day 2; Mesna: g/m2, day 2; Carboplatin: AUC 5, day 2; Etoposide: 100 mg/m2, days 1-3] from September 2010 to January 2011. PET Scan restaging Post 6 cycles of ICE chemo showed residual disease T cell NHL with same Stage IIIB which warranted further management (Figure 1-4).
Investigations
PET Scan restaging Post 6 cycles of ICE showed bilateral cervical lymph node level I B ,II, IV, V (SUV 4.15gm/ml). Bilateral Axillary lymph node (SUV 3.22 gm/ml) & Mediastinal lymph node(Pre vascular lymph nodes sub cranial lymph node) (3.71 gm/ml). Retro peritoneal lymph node (Para aortic SUV 3.03gm/ml , aorto caval SUV 3.14gm/ml). Bilateral Iliac and Bilateral Inguinal LN present. Spleen SUV 4.gm/ml and Bone marrow SUV 3gm/ml (Figure 1-4).
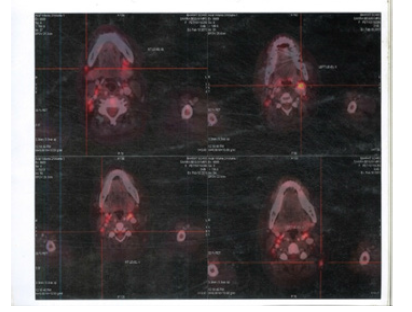
Figure 1:PET CT 10th Feb 2011 showing Axial Sections Cervical FDG Avid Lymph nodes
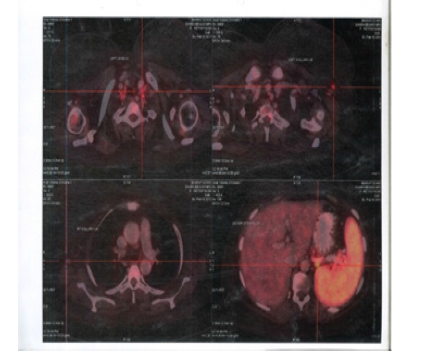
Figure 2:PET CT 10th Feb 2011 showing Axial Sections FDG Avid Mediastinal Lymph nodes and Spleen
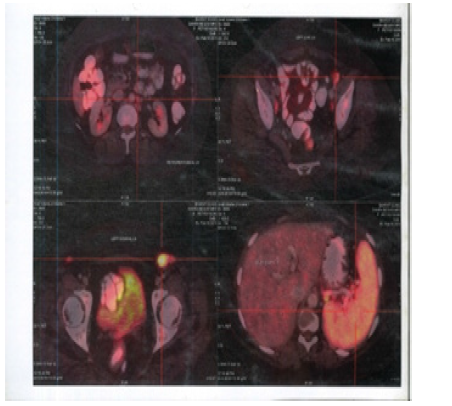
Figure 3:PET CT 10th Feb 2011 showing Axial Sections FDG Avid Abdominal Lymph nodes and Spleen
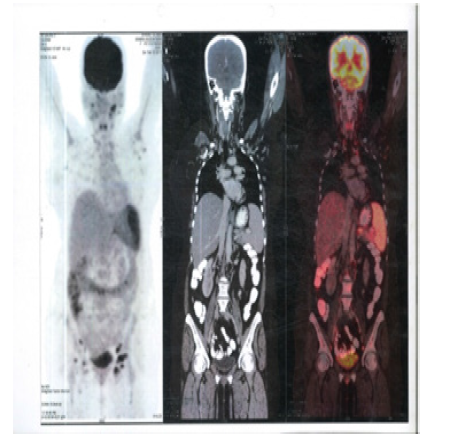
Figure 4:PET CT 10th Feb 2011 showing Coronal Sections FDG Avid Whole body FDG Avid lesions
Treatment
Patient was treated with Mantle field (Bilateral cervical, Supraclaclavicular, Infraclavicular, Mediastinal, Hilar and Axilla lymph nodes) 45Gy @ 1.8Gy in 25 fraction March to April 2011. Inverted Y ( Paraaortic, Pelvic and Inguinal field as well as upper femoral nodes) with splenic field 45Gy @ 1.8Gy in 25 fraction May to July 2011. Post Radiation PET CT in September 2012 showed complete resolution of the metabolically active lymph nodes in the neck, axillae, abdomen and inguinal regions. Splenic uptake resolved however residual lesions with metabolically active sclerotic marrow lesions in the Iliac bones, D9 vertebra and Proximal humeri and femora were seen (Figure 5-8). Hence in September 2011 a boost of 24 Gy in 1.8 Gy per fraction was given to the Proximal left and right humerus, Bilateral Iliac boost including the bilateral femoral heads and D9 Spine were given. Post Radiation follow up PET CT was taken in September 2012 which was normal (Figure 9-10).
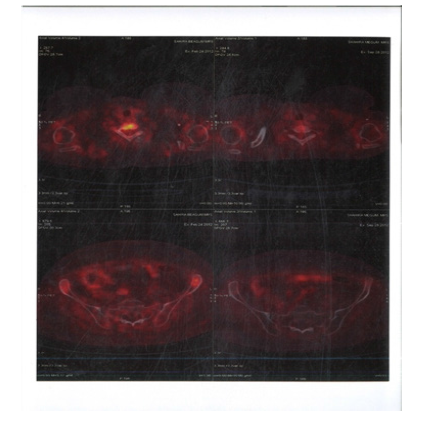
Figure 5:PET CT 28th September 2012 showing Axial Sections no FDG Avid Abdominal Lymph nodes and Spleen
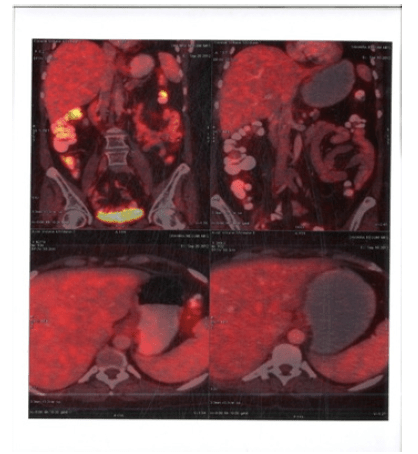
Figure 6:PET CT 28th September 2012 showing Coronal Sections Abdomen no FDG Avid lesions
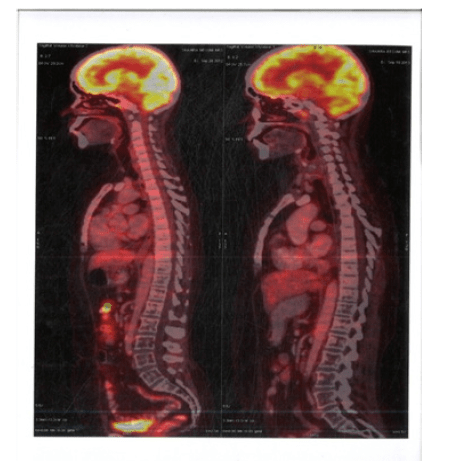
Figure 7:PET CT 28th September 2012 showing Sagittal Sections Abdomen no FDG Avid lesions
Figure 8:PET CT 28th September 2012 showing Coronal Sections Whole body no FDG Avid lesions
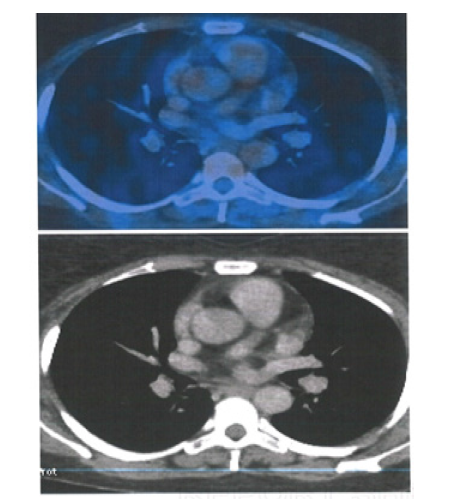
Figure 9:PET CT 20th May 2016 showing Axial Sections no FDG Avid Abdominal Lymph nodes and Spleen
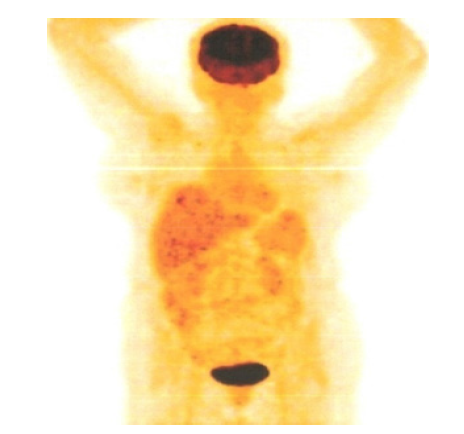
Figure 10:PET CT 20th May 2016 showing Coronal Sections Whole body no FDG Avid lesions
Outcome and follow-up
Since then Patient has been on follow up last 10 years. The serial PET CT scans taken are normal and the patient is in complete remission last 10 years. On follow up she has been having chipping of teeth and mild pain in both the hips. On Investigation she is diagnosed to have, avascular necrosis bilateral femur heads. She has been on regular thyroid supplements for hypothyroidism. Her echocardiogram showed global hypokinesia in 2016. In 2020 January Echocardiogram showed Mild Left ventricular dysfunction with Ejection fraction 45%. Her LDH was 151U/ml. Her hematologic parameters and blood chemistry parameters are normal Her Activities of Daily Living (ADLs) is unimpaired. The PET CT taken in January 2020 shows no evidence of disease (Figure 11-14). She continues follow up visits.

Figure 11:PET CT 7th Jan 2020 showing Axial Sections cervical region No FDG Avid lesions
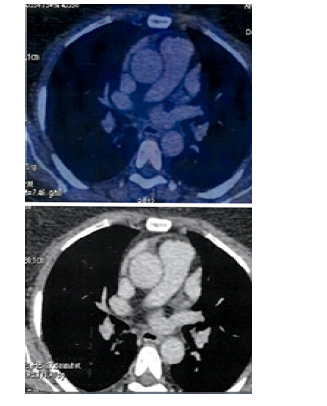
Figure 12:PET CT 7th Jan 2020 showing Axial Sections Mediastinum No FDG Avid lesions
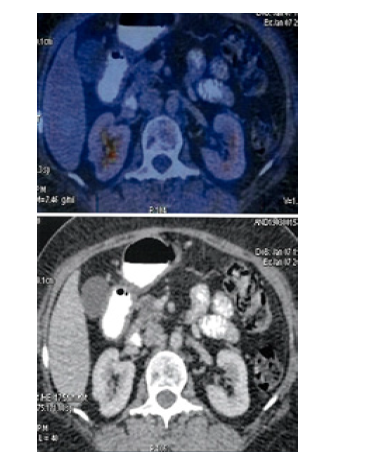
Figure 13:PET CT 7th Jan 2020 showing Axial Sections Abdomen No FDG Avid lesions
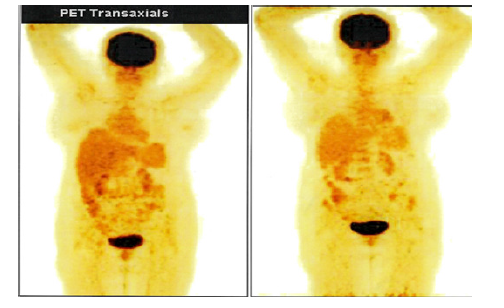
Figure 14:PET CT 7th Jan 2020 showing Coronal Sections Whole body no FDG Avid lesions
Discussion
Peripheral T-cell lymphoma (PTCL) is aggressive and is classified as a subtype of non-Hodgkin’s lymphoma (NHL). PTCL encompasses a diverse group of lymphomas. PTCL as a cancer arises in the lymphoid tissues outside the bone marrow such as lymph nodes, gastrointestinal tract, spleen and skin. Its Incidence is 10 -15% of all NHL types. Peripheral T-cell lymphomas (PTLs) have an extremely poor prognosis especially when patients relapse or are refractory to conventional chemotherapy. The most common subtype is called PTCL-not-otherwise specified (PTCL-NOS) mostly prevalent in Europe and North America ; Anaplastic large cell lymphoma (ALCL) in Europe and North America; Angioimmunoblastic T-cell lymphoma (AITL) in Europe; NK-/ T-cell lymphoma (NKTCL) and adult T-cell leukemia (ATLL) in Asia [4,5]. There are many other subtypes as well.
Treatment Options
1. In the first line, the survival outcomes of CHOP in PTCL have been promising and are explicitly illustrated in the ITCP and BCCA series. Hence our patient was given CHOP [4,5].
2. CHOEP for 6 cycles (etoposide was omitted for patients >60 years of age) was given to Nordic study by d’Amore patients . The patients who attained complete response or partial response proceeded to high-dose therapy with carmustine, etoposide, cytarabine, and melphalan (or cyclophosphamide) and ASCT [6].
3. The second largest prospective study was conducted by Reimer and colleagues evaluating ASCT in first remission after CHOP in 83 patients [7].
4. CHOP-based regimen was preferred, followed by consolidation with ASCT for those with PTCL-NOS, AITL, and ALK-negative ALC [8]. There are many options for the salvage treatment but the availability to all socioeconomic groups is restricted. The different options in a salvage setting of PTCL are
5. Other chemotherapy lines such as ICE, cisplatin in combination with high dose Ara-C and dexamethasone, DHAP,ESHAP, Gemcitabine, single-agent lenalidomide [9-13].
6. Romidepsin [14].
7. Single-agent brentuximab vedoti [15].
8. Single-agent Belinostat (PXD101) [16].
9. Single-agent Pralatrexate [17].
10. Salvage Radiotherapy [17].
11. Immunotherapy [18].
12. Allogeneic hematopoietic stem cell transplant [19].
13. Alemtuzumab, a humanized anti-CD52 monoclonal antibody, as therapy for patients with heavily pretreated and refractory PTL [4,20,21].
14. (CAR) T cell [1,22-24].
15. The aim for allogeneic stem cell transplant (alloSCT) in fit patients, has been more reliably curative than ASCT in the relapsed setting [25].
Salvage radiation therapy
With evolving new drugs in the armamentarium of treatment for PTCL we often tend to underestimate those treatment modalities which have been used in the yester years. Radiation in the form of Total Nodal radiation (TNI) had been used rampantly three decades ago as an important treatment modality in Hodgkins Lymphoma or non hodgkins lymphoma, prior to the use of chemotherapeutic agents [26, 30]. Due to the long term toxicities and the advent of new drugs, radiation has lost its place in many settings. Total Nodal radiation has decreased the usage of costly immunosuppressive drugs and chemotherapeutic agents in relapsed lymphomas.
In comparison to DLBCL, T-cell NHL presents more commonly with stage III and IV disease (48% and 74%, respectively) and T-cell patients frequently present with combined nodal as well as extranodal disease at diagnosis, thereby the need for the addition of radiation is mandatory. Studies specifically addressing the benefits of radiation therapy to bulky sites for patients with advanced T-cell NHL have not been performed [27-30].
Historically, Radiation was known to cause risks of late toxicities and second malignancies in long-term Survivors [27-30]. Radiation dose to heart has a linear association with the risk of cardiac mortality increases with mediastinal radiation dose and the incidence increases by 60% for every 1 Gy. The cumulative incidence of breast cancer at age 50 years was 35% among Hodgkins Lymphoma survivors’ females who received chest irradiation for their childhood cancer diagnosis (mantle field given between 1970 and 1986) [27,28]. It can be reiterated that the late-toxicity figures apply to patients who were treated for childhood cancers with ancient techniques with Cobalt 60 machine with 2 dimensional (2D) planning systems. One can never project the same toxicity profile to adult patients treated during these days with modern radiation methods. The latest innovation of 3D conformal radiation (3DCRT), Intensity Modulated radiation (IMRT), Image guided radiation (IGRT), and volumetric modulated arc therapy (VMAT) refinements have been recently described, to optimize dose delivery and efficiently spare the organ at risk. The latest radiation modalities and standards should translate into reduced toxicity. However there are not many studies on salvage radiation and their long term developments due to the advent of newer drugs. In a resource limited setting radiation comes in handy to get the same consistent effects as salvage chemo drugs / bone marrow transplant with limited/acceptable toxicity and more studies are needed to elucidate the same [29,30].
In this case the use of radiation was preferred over Stem cell transplant and other new drugs due to financial constraints. This patient has benefited well with toxicities which are tolerable with a sustained remission.
Learning points/take home messages
1. We often tend to restrict our management based on the current guidelines given. In resource limited setting, it becomes imperative to think out of the box to make tailor-made decisions which will suit individual patients.
2. The outcome of this patient is promising, reminding us that the old (modality) can still remain gold in the management of some patients even if not all.
3. Therefore we should give space for the so called obsolete methods in oncology in selective cases.
References
1. Foss F. Hematology: relapsed and refractory PTCL±into the therapeutic abyss. Nat Rev Clin Oncol. 2011; 8:321±322.
2. National Comprehensive Cancer Network: NCCN clinical practice guideline on oncology: Non-Hodgkin's lymphomas, 2020.
3. Bachy E, Coiffier B. Time has come for immunotherapy in PTCL. Blood. 2014; 123:3059±3060.
4. Savage KJ, Chhanabhai M, Gascoyne RD, et al. Characterization of peripheral T-cell lymphomas in a single North American institution by the WHO classification. Ann Oncol. 2004;15(10):1467-1475.
5. Vose J, Armitage J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26(25):4124-4130.
6. d'Amore F, Relander T, Lauritzsen GF, et al. Up-front autologous stem-cell transplantation in peripheral T-cell lymphoma: NLG-T-01. J Clin Oncol. 2012;30(25):3093-3099
7. Reimer P, Rudiger T, Geissinger E, et al. Autologous stem-cell transplantation as first-line therapy in peripheral T-cell lymphomas: results of a prospective multicenter study. J Clin Oncol. 2009;27(1):106-113.
8. Mehta N, Maragulia JC, Moskowitz A, et al. A retrospective analysis of peripheral T-cell lymphoma treated with the intention to transplant in the first remission. Clin Lymphoma Myeloma Leukemia. 2013;13(6):664-670.
9. Zelenetz AD, Hamlin P, Kewalramani T, et al. Ifosfamide, carboplatin, etoposide (ICE)-based second-line chemotherapy for the management of relapsed and refractory aggressive non-Hodgkin’s lymphoma. Ann Oncol. 2003; 14(suppl 1):i5-i10. pmid:12736224.
10. Reddy NM, Evens AM. Chemotherapeutic advancements in peripheral T-cell lymphoma. Semin Hematol. 2014;51:17-24. pmid:24468312.
11. Velasquez WS, Cabanillas F, Salvador P, et al. Effective salvage therapy for lymphoma with cisplatin in combination with high dose Ara-C and dexamethasone (DHAP). Blood. 1998; 71:117-122.
12. Velasquez WS, McLaughlin P, Tucker S, et al. ESHAP–an effective chemotherapy regimen in refractory and relapsing lymphoma: a 4-year follow-up study. J Clin Oncol. 1994;12:1169-1176. pmid:8201379
13. Zinzani PL, Venturini F, Stefoni V, et al. Gemcitabine As single agent in pretreated T-cell lymphoma patients: evaluation of the long-term outcome. Ann Oncol. 2010; 21:860–863. pmid:19887465
14. Morschhauser F, Fitoussi O, Haioun C, Thieblemont C, Quach H, Delarue R, et al. A phase 2, multicentre, single-arm, open-label study to evaluate the safety and efficacy of single-agent lenalidomide (Revlimid) in subjects with relapsed or refractory peripheral T-cell non-Hodgkin lymphoma: the EXPECT trial. Eur J Cancer. 2013; 49:2869-2876. pmid:23731832
15. Coiffier B, Pro B, Prince HM, et al. Results from a pivotal, open-label, phase II study of romidepsin in relapsed or refractory peripheral T-cell lymphoma after prior systemic therapy. J Clin Oncol. 2012;30(6):631-636.
16. Horwitz SM, Advani RH, Bartlett NL. Objective responses in relapsed T-cell lymphomas with single-agent brentuximab vedotin. Blood. 2014; 123:3095-3100. pmid:24652992
17. Foss F, Advani R, Duvic M, et al. A Phase II trial of Belinostat (PXD101) in patients with relapsed or refractory peripheral or cutaneous T-cell lymphoma. Br J Haematol. 2015;168:811-819. pmid:25404094
18. O'Connor OA, Pro B, Pinter-Brown L, et al. Pralatrexate in patients with relapsed or refractory peripheral T-cell lymphoma: results from the pivotal PROPEL study. J Clin Oncol. 2011;29:1182-1189. pmid:21245435
19. Bachy E, Coiffier B. Time has come for immunotherapy in PTCL. Blood 2014; 123:3059-3060. pmid:24832935
20. Goldberg JD, Chou JF, Horwitz S, et al. Long-term survival in patients with peripheral T-cell non- Hodgkin lymphomas after allogeneic hematopoietic stem cell transplant. Leuk Lymphoma. 2012;53:1124-1129. pmid:22136377
21. Enblad G, Hagberg H, Erlanson M, et al. A pilot study of alemtuzumab (anti-CD52 monoclonal antibody) therapy for patients with relapsed or chemotherapy-refractory peripheral T-cell lymphomas. Blood. 2004; 103:2920-2924. pmid:15070664
22. Kim SJ, Kim K, Park Y, et al. Dose modification of alemtuzumab in combination with dexamethasone, cytarabine, and cisplatin in patients with relapsed or refractory peripheral T-cell lymphoma: analysis of efficacy and toxicity. Invest New Drugs. 2012;30:368–375. pmid:20734108
23. Brandon S. Imber Michel Sadelain,Carl DeSelm, et al. Early experience using salvage radiotherapy for relapsed/refractory non-Hodgkin lymphomas after CD19 chimeric antigen receptor (CAR) T cell therapy. Brit J Hematol. First published: 05 March 2020 https://doi.org/10.1111/bjh.16541
24. Cheah CY, Oki Y, Fanale MA. Novel treatments for T-cell lymphoma. Am Soc Clin Oncol Educ Book. 2015; 35:e468–478. pmid:25993211
25. Karlin L, Coiffier B. The changing landscape of peripheral T-cell lymphoma in the era of novel therapies. Semin Hematol. 2014;51:25-34. pmid:24468313
26. Lunning MA, Moskowitz AJ, Horwitz S. Strategies for relapsed peripheral T-cell lymphoma: the tail that wags the curve. J Clin Oncol. 2013;31(16):1922-1927.
27. Melnyk A, Rodriguez A, Pugh WC, et al. Evaluation of the revised European-American Lymphoma classification confirms the clinical relevance of immunophenotype in 560 cases of aggressive non-Hodgkin’s lymphoma. Blood. 1997; 89:4514-4520
28. Schaapveld M, Aleman BM, van Eggermond AM, et al. Second cancer risk up to 40 years after treatment for Hodgkin’s lymphoma. N Engl J Med. 2015; 373(26):2499-511.
29. van Nimwegen FA, Schaapveld M, Cutter DJ, et al. Radiation dose-response relationship for risk of coronary heart disease in survivors of Hodgkin lymphoma. J Clin Oncol. 2016; 34(3):235-43.
30. Gustafsson A. Non-Hodgkin's Lymphomas (NHL), Acta Oncologica. 1996; 35:sup7, 102-116, DOI: 10.3109/02841869609101670.
31. Hoskin PJ, Díez P, Williams M, et al. Recommendations for the Use of Radiotherapy in Nodal Lymphoma. Clin Oncol. 2013; 25 (2013) 49e58.
Received: May 23, 2021;
Accepted: June 21, 2021;
Published: June 23, 2021.
To cite this article : Daniel JM, Ramesh A , Kalaichelvi K. Non Hodgkin’s Lymphoma T cell (PTCL-NOS) Post Salvage Radiation, a Long Term Survivor. British Journal of Cancer Research. 2021; 4:1.
©2021 Daniel JM, et al.
