Research article / Open Access
DOI: 10.31488/bjcr.166
Perioperative Progress and Predictor of the Postoperative Mental Quality of Life for Lung Cancer Patients
Ryuta Fukai1,3, Tomoki Nishida1, Yuto Igarashi2, Takaaki Murata2, Yuma Sunoh2, Katsunori Miyake2, Naoko Isogai2, Rai Shimoyama2, Jun Kawachi2, Hiroyuki Kashiwagi2
1. Department of Thoracic Surgery, Shonan Kamakura General Hospital, Kamakura, Japan
2. Department of Surgery, Shonan Kamakura General Hospital, Kamakura, Japan
3. Department of Thoracic Surgery, Dokkyo Medical University, Mibu, Japan
*Corresponding author: Ryuta Fukai, Department of Thoracic Surgery, Shonan Kamakura General Hospital, 1370-1, Okamoto, Kamakura, 247-8533, Japan
Abstract
Background: It seems that the measurement of quality of life for lung cancer patients are becoming important because the age of the patients are being higher in recent years and the magnitude of the surgical stress for lung cancer relatively appears to be greater compared with other malignancies. Methods: We prospectively investigated perioperative (before, 1, 3, 6, and 12 months after surgery) mental quality of life for non-small lung cancer patients who received anatomical pulmonary resection at our institution using the Short Form Health Survey 36. Results: Preoperatively there were significant differences of mental quality of life scores regarding smoking status and life-style. It was about 3 to 6 months that the patients needed the duration for the postoperative recovery of mental quality of life compared with the baseline. In univariate analysis, there were significant differences between mental quality of life at 6 months after surgery, concerning smoking status (current vs not current, p=0.0267), mode of living (alone vs not alone, p=0.017), and charlson comorbidity index (≧3 vs <3, p=0.00165). In multivariate analysis, the patients living alone (p=0.01797) and with more comorbid status (charlson comorbidity index≧3, p=0.00131) showed significantly worse mental quality of life at 6 months after surgery. Conclusions: Living in solitude and more comorbid status were independent significant predictor of the worse mental quality of life at 6 months after surgery. Additional social or psychological support might be considered for such patients who received anatomical lung cancer surgery to maintain their mental condition.
Key words: mental quality of life, lung cancer, surgery, smoking status, charlson comorbidity index, solitude
Introduction
Lung cancer is still the leading cause of cancer-related mortality worldwide in the both sexes. Surgery is the most effective treatment for early-stage lung cancer diagnoses and considered the best curative option. However, it is the more invasive treatment compared with other among treatment modalities, such as chemotherapy or radiotherapy. Moreover, surgery substantially impacts quality of life (QOL) of the patients comparatively long time [1].
It has been reported QOL of lung cancer patients more profoundly worsen and for a longer time compared with other cancer patients [2], and health-related QOL is significantly associated with overall survival [1]. On the other hand, in Japan, the age of non-small cell lung cancer (NSCLC) patients who receive surgery has been aging, and the rate of early-stage disease has been increased recently [3,4]. Therefore, we think that evaluating perioperative QOL of NSCLC patients is increasingly becoming important. However, most of the literatures that reported recovery of QOL after lung cancer surgery had evaluated it only before surgery and once postoperatively. There are few papers describing the frequent long-time QOL development of NSCLC patients who underwent anatomical pulmonary resection.
In several studies, it has been revealed that mental health status correlates with perioperative clinical course [1,2,5,]. Preoperative mood status of the NSCLC patients evaluated through Psychological Global Well Being Index significantly correlated with the postoperative mental QOL (M-QOL) [2]. In addition, it is reported that comprehensive psychological intervention can effectively relieve pain, improve immune functions and enhance quality of life for patients suffering from lung cancer surgery [6].
In this study, we investigated socio-demographic factors of NSCLC patients who underwent anatomical pulmonary resection with curative intent in our hospital to ascertain recovery time of M-QOL from surgery and predictors of perioperative M-QOL.
Materials and Methods
This is a prospective longitudinal study of 87 consecutive cases who diagnosed of primary pulmonary malignancy and underwent surgery from April 2015 to November 2017. The patients who underwent partial resection, re-do pulmonary resection for lung cancer, refused to cooperate in this study, and was difficult to continue their questionnaire postoperatively due to major complication were excluded. We investigated the relationship between pre and postoperative (6 months after surgery) M-QOL and patient’s characteristics, which included age, gender, smoking status, mode of living, and Charlson comorbidity index (CCI). We obtained written informed consent from the all participated patients who underwent anatomical pulmonary resection at our institution. The study was approved by our Institutional Review Board (approval number SKEC-17-A1).
Quality of life assessment
QOL was assessed using the Japanese version of the Short Form Health Survey 36 (SF-36) [7]. The patient administered the questionnaire one day before surgery and at 1, 3, 6 and 12 months after surgery on a consultation day as an outpatient.
SF-36
The SF-36 is a self-rating questionnaire composed of 36 items, grouped into eight scales, which include both physical and mental health and assesses eight dimensions of quality of life. In this study, we evaluated only mental health which comprise of four subscales in this study; mental health (MH), role-emotional (RE), self-functioning (SF), and vitality (VT). The raw scale are standardized and range 0 to 100 where 0 represents the poorest state of health and 100 the best possible. The nation standard level (NSL) with confidence interval of 95% was used for considering the status of mental health. The reliability and validity of the SF-36 questionnaires have been confirmed in international cancer studies [8-11].
Charlson comorbidity index
Each patient was scaled in the Charlson comorbidity index (CCI). Patients were considered to have a comorbid status if a listed disease was confirmed in the medical records or if the patient was treated for it. The modified CCI was used, as proposed by Birim et al. [12].
Smoking status and living conditions
Smoking status which had heard from each patient was classified into three groups: never smokers, former smokers (stopped smoking before more than 1 year), and current smokers (continued to smoke or stopped smoking within 1 year). Living conditions were classified into two groups: living alone, and living with somebody.
Statistical analysis
In agreement with procedures by the SF-36, scores were linearly converted to a scale ranging from 0 to 100 for each patient. For the global/QOL and functional scales, higher scores represent a higher level of functioning. For the symptom scales, higher scores represent a greater symptom burden. Results were reported as mean. To evaluate the relationship between clinical factors and M-QOL, the mean score of the four subscales in the all patients was used. Statistical analysis was performed using R for windows (R 3.6.1). The Student’s t-test was used to perform preoperative correlation calculations between clinical factors and SF-36 scores and to evaluate the correlation between clinical factors and SF-36 scores at 6 months after surgery. Logistic regression analysis was used in multivariate analysis.
Results
The preoperative response rate to the QOL questionnaire was 100%, at 1 month 94%, at 3 months 97%, at 6 months 91%, at 12 months 89%. Socio-demographic and clinical characteristics of the patients are showed in Table 1.
Table 1:Socio-demographic and clinical characteristics of patients (N=87)
| Characteristic | Number (%) |
|---|---|
| Age (years) | |
| Mean | 69.7 ± 8.5 |
| Range | 48-83 |
| Sex | |
| Male | 41(47) |
| Female | 46(53) |
| Smoking status | |
| Never | 40(46) |
| Former | 30(34) |
| Current | 17(20) |
| Mode of living | |
| Alone | 10(11) |
| Not alone | 77(89) |
| Charlson comorbidity index | |
| <3 | 77(89) |
| ≥3 | 10(11) |
| Surgery | |
| Segmentectomy | 73(84) |
| Squamous cell carcionoma | 12(14) |
| Adenosquamous | 1(1) |
| Carcinoid | 1(1) |
| p-Stage | |
| IA | 35(40) |
| IB | 27(31) |
| IIA | 7(8) |
| IIB | 12(14) |
Baseline M-QOL subscales
Baseline (preoperative) M-QOL was comparable to NSL (50%) in the four subscales (Figure 1). There were significant differences statistically in the mean value of four subscales in M-QOL between current smokers and others (p=0.04284), and between living alone and others (p=0.004424) (Table 2).
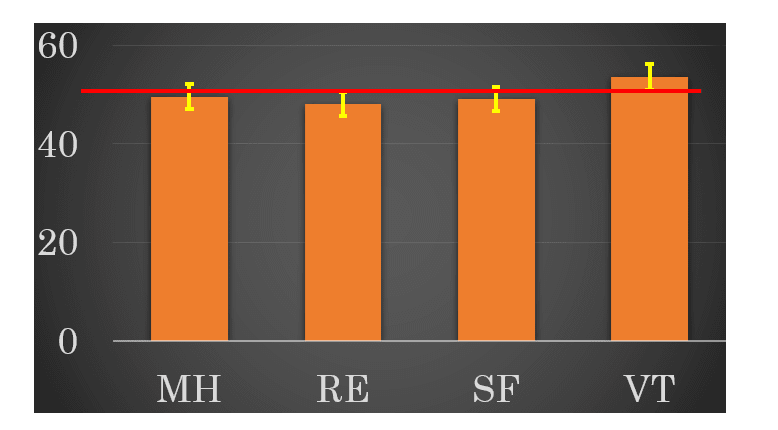
Figure 1:Preoperative mental health which comprise of four subscales.
Table 2:Preoperative analysis between clinical factors and mental quality of life
| Variables | No. of Patients | M-QOL score (mean±SD) | p value |
|---|---|---|---|
| Age | 0.3 | ||
| <75years | 60 | 50.73±8.20 | |
| ≥75years | 27 | 48.06±10.72 | |
| Sex | 0.6 | ||
| Male | 41 | 50.42±10.14 | |
| Female | 46 | 49.44±8.11 | |
| Smoking status | 0.0 | ||
| Not current | 70 | 51.02±8.52 | |
| Current | 17 | 45.31±10.10 | |
| Mode of living | 0.0 | ||
| Alone | 10 | 40.49±8.97 | |
| Not alone | 77 | 51.13±8.40 | |
| Charlson comorbidity index | 0.5 | ||
| <3 | 77 | 49.57±8.49 | |
| ≥3 | 10 | 52.45±13.06 |
Postoperative M-QOL evolution of after surgery
In M-QOL evolution of the four subscales, all subscales showed significantly decrease at 1 month after surgery. MH and VT recovered to baseline at 3 months after surgery, and the other two scales (RE, SF) also recovered to baseline at 6 months after surgery (Figure 2).
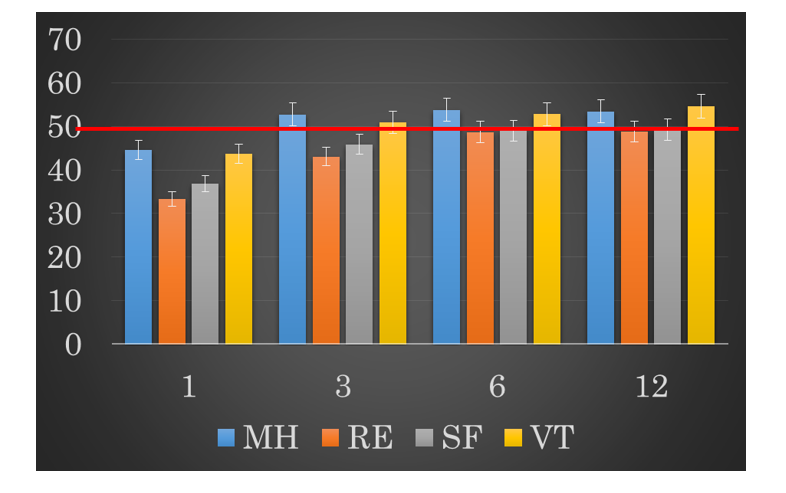
Figure 2: Postoperative development of four subscales of mental quality of life.
Predictor of postoperative M-QOL
We analyzed the association between patient’s characteristics and postoperative M-QOL, which had almost recovered at 6 months after surgery compared with baseline M-QOL.
Among the evaluated factors, there were significant differences between M-QOL of the four subscales (mean ± standard deviation) at 6 months after surgery regarding smoking status (current vs not current, 45.54±11.28 vs 51.30±7.96, p = 0.0267), mode of living (alone vs not alone, 44.08±12.24 vs 51.18±7.96, p = 0.017), and CCI (≥3 vs <3, 41.13±14.20 vs 51.31±7.49, p = 0.00165) (Table 3).
Table 3:Univariate analysis of factors associated with mental quality of life
| Variables | No. of Patients | M-QOL score (mean±SD) | p value | |
|---|---|---|---|---|
| Age | ||||
| <75years | 60 | 50.36±8.78 | 0.9 | |
| ≥75years | 27 | 50.02±9.21 | ||
| Sex | ||||
| Male | 41 | 49.55±9.56 | 0.5 | |
| Female | 46 | 50.81±8.33 | ||
| Smoking status | 0.0 | |||
| Not current | 70 | 51.30±7.96 | ||
| Current | 17 | 45.54±11.28 | ||
| Mode of living | 0.0 | |||
| Alone | 10 | 44.08±12.24 | ||
| Not alone | 77 | 51.18±7.96 | ||
| Charlson comorbidity index | 0.0 | |||
| <3 | 77 | 51.31±7.49 | ||
| ≥3 | 10 | 41.13±14.20 | ||
| SD: standard deviation | ||||
Moreover, in multivariate analysis among these three factors, mode of living (95% confidence interval (95% CI) 1.15-11.88, P = 0.01797) and CCI (95% CI -15.74--3.98, p = 0.00131) showed significant differences on M-QOL at 6 months after surgery (Table 4).
Table 4:Multivariate analysis of factors associated with mental quality of life at 6 months after surgery
| Variables | No. of Patients | p value (95% confidence interval) |
|---|---|---|
| Smoking status | 0.1 | |
| Not current | 70 | (-9.31-0.06) |
| Current | 17 | |
| Mode of living | 0.0 | |
| Alone | 10 | (1.15-11.88) |
| Not alone | 77 | |
| Charlson comorbidity index | 0.0 | |
| <3 | 77 | (-15.74--3.98) |
| ≥3 | 10 |
Discussion
It has been reported QOL of lung cancer patients more profoundly worsen and for a longer time compared with other cancer patients [2], and health-related QOL is significantly associated with overall survival [1]. On the other hand, in Japan, the age of NSCLC patients who receive surgery has been aging, and the most of them have had early-stage disease [3,4]. Under these situations, it seems that the perioperative QOL evaluation of NSCLC patients is increasingly becoming important. We conducted prospective study, in which we performed five times perioperative M-QOL evaluation of NSCLC patients using SF-36, and found that there were significant differences in preoperative M-QOL of the NSCLC patients regarding smoking status and mode of living, and the M-QOL of them had recovered approximately six months after surgery. Furthermore, we identified that living alone (p=0.01797), and comorbidity status (CCI≧3, p=0.00131) were independent predictors of postoperative lower M-QOL.
In most of previous studies, the questionnaires were administered only one or two times [8,9,13], while in the present study, the questionnaires were administered five times (before surgery, and 1,3,6, and 12 months postoperatively), similar to another report [14]. Therefore, we believe that our results is more accurate for evaluating recover period after curative pulmonary resection, it was from 3 to 6 months after surgery. In preoperative M-QOL analysis, there were significant differences in smoking status and mode of living. Further, mode of living was also independent predictor of worse postoperative M-QOL (p=0.01797), and smoking status showed a tendency of worse postoperative M-QOL (p=0.5302). From these results, it seems that the patients who lives alone and smokes currently or stopped smoking within 1 year are easier to decrease postoperative M-QOL, therefore, we may adopt additional strategy. Zhao et al. reported that comprehensive psychological intervention can effectively relieve pain, improve immune functions and enhance quality of life for patients suffering from lung cancer surgery [6]. In future, we might consider doing beforehand psychological intervention for the lung cancer patients who have lived alone or currently smoked preoperatively.
In this study, there was a significant difference between patients living alone and other patients on both pre and postoperative M-QOL. It has been reported that mode of life appears to affect both the survival and QOL of cancer patients [19,20]. Han KT et al. demostrated that there was significant relationship between marital status and QOL, and the multilevel analysis by marital status showed that single men had significantly worse QOL than married men [20]. Moreover, Jatoi A et al. reported that widowed and married patients scored better on the domains of spirituality, support of family and friends, and overall lung cancer symptoms [21]. From these results, living alone may exacerbate mental condition and clinical outcomes of the patients who might become negative for receiving cancer treatment because the support of family or housemate may influence not only daily life but also the type of cancer treatment of them. Thus, we should continue to remain sensitive to the mode of life (living alone or not) of the patients when we care them.
The CCI was developed by Charlson et al. [22], and it has been reported that CCI is strongly correlated with higher risk of surgery in NSCLC patients [12]. In the prostate cancer patients, it has been noted that CCI seems useful mainly in predicting long-term QOL and physical function scores [23]. In our study, it was proved that CCI is a predictor of postoperative M-QOL for NSCLC patients. To our knowledge, this is the first report which documented the relationship between CCI and QOL status in lung cancer patients. When we reviewed the results of questionnaire in our patients who had more comorbidity (CCI≧3), remarkable deterioration of work, usual activity, mental condition, and subjective feeling of health were observed in some representative patients at 6 months after surgery compared to the baseline. From our results, medical staffs should pay attention to M-QOL of NSCLC patients with higher CCI postoperatively.
Smoking and QOL was found to have negative association in many studies that utilize QOL measures in active smokers [9,15]. Our result that the QOL of current smokers was worse than that of former and never smokers, seems to reflect those reports. However, in preoperative duration of lung cancer surgery, there are few studies that reported the significant difference of M-QOL due to smoking status, therefore we consider our result have certain worth. It is reported that smoking lower human QOL in the individuals with comorbidity as well as in healthy people [16], moreover, negative association between smoking and QOL has been identified across nations, and diverse socioeconomic and cultural groups [15]. Balduyck et al. reported smoking cessation is beneficial at any time point to lung cancer surgery and current smoking at the time of surgery is associated with a poor postoperative QOL [17]. In addition, Hays et al. reported that medications (such as varenicline and bupropion SR) provide both a direct and indirect effect through continuous smoking abstinence [18]. From these studies, we might conduct smoking cessation treatment with pharmacotherapy in preoperative period to prevent having poor postoperative QOL.
A limitation of the present study was the small number of patients, although the preoperative mode of life and CCI were significant predictor of postoperative M-QOL. An additional limitation was that this was a single-institutional study.
In conclusion, M-QOL of NSCLC patients who had received anatomical pulmonary resection recovered at 3 to 6 months after surgery. Living alone and higher CCI (≧3) were independent predictors of worse M-QOL postoperatively. Therefore, such patients might need to receive some additional support for maintaining their mental condition and real life. Further investigation will be needed to determine the best timing and method of the intervention for these patients.
Abbreviations
QOL:quality of life; NSCLC:non-small cell lung cancer; M-QOL:mental QOL; CCI:Charlson comorbidity index; SF-36:Short Form Health Survey 36; MH:mental health; RE: role-emotional; SF: self-functioning; VT: vitality; NSL:nation standard level; CI: confidence interval; SD:standard deviation
Acknowledgements
The authors gratefully thank Masahiro Hirata for his contribution to the statistical analysis, and Etsuko Shimizu and Keiko Asou for their contribution for the management of questionnaire.
Conflicts of Interest
None declared.
References
1. Poghosyan H, Sheldon LK, Leveille SG, et al. Health-related quality of life after surgical treatment in patients with non-small cell lung cancer: a systematic review. Lung Cancer. 2013;81(1):11-26.
2. Barlési F, Doddoli C, Loundou A, et al. Preoperative psychological global well being index (PGWBI) predicts postoperative quality of life for patients with non-small cell lung cancer managed with thoracic surgery. Eur J Cardio-thorac Surg. 2006;30(3):548-53.
3. Ueda Y, Fujii Y, Kuwano H. Thoracic and cardiovascular surgery in Japan during 2007: annual report by the Japanese association for thoracic surgery. Gen Thorac Cardiovasc Surg. 2009;57(9):488-513.
4. Committee for scientific affairs, The Japanese association for thoracic surgery, Okada M, et al. Thoracic and cardiovascular surgeries in Japan during 2017. Annual report by the Japanese association for thoracic surgery. Gen Thorac Cardiovasc Surg.2020;68(4):414-49.
5. Sarna L, Padilla G, Holmes C, et al. Quality of life of long-term survivors of non-small )cell lung cancer. J Clin Oncol. 2002; 20(13): 2920-29.
6. Zhao X, Cui L, Wang W, et al. Influence of psychological intervention on pain and immune functions of patients receiving lung cancer surgery. Park J Med Sci. 2016;32(1):155-9.
7. McHorney CA, Ware JE, Lu JF, et al. The MOS 36-item short-form health survey (SF-36): Ⅲ. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32(1): 40-66.
8. Möller A, Sartipy U. Predictors of postoperative quality of life after surgery for lung cancer. J Thorac Oncol. 2012;7(2): 406-11.
9. Myrdal G, Valtysdottir S, Lambe M, et al. Quality of life following lung cancer surgery. Thorax. 2003;58(3):194-7.
10. Sarna L, Cooley ME, Brown JK, et al. Womwn with lung cancer: quality of life after thoracotomy: a 6-month prospective study. Cancer Nurs. 2010;33(2):85-92.
11. Ostroff JS, Krebs P, Coups EJ, et al. Health-related quality of life among early-stage, non-small cell, lung cancer survivors. Lung Cancer 2011;71(1):103-8.
12. Birim Ö, Matt APWM, Kappetein AP, et al. Validation of Charlson comorbidity index in patients with operated primary non-small cell lung cancer. Eur J Cardio-thorac Surg. 2003:23(1):30-4.
13. Pompili C, Brunelli A, Xiumé F, et al. Predictors of postoperative decline in quality of life after major lung resections. Eur J Cardio-thorac Surg. 2011;39(5):732-7.
14. Balduyck B, Hendriks J, Lauwers P, et al. Quality of life evolution after lung cancer surgery in septuagenarians: a prospective study. Eur J Cardio-thorac Surg. 2009;35(6):1070-5.
15. Goldenberg M, Danovitch I, IsHak WW. Quality of life and smoking. Am J Addict. 2014;23(6):540-62.
16. Efendi V, Özalevli S, Naz i, et al. The effect of smoking on body composition, pulmonary function, physical activity and health-related quality of life among healthy women. Tuberk Toraks. 2018;66(2):101-8.
17. Balduyck B, Nia PS, Cogen A, et al. The effect of smoking cession on quality of life after lung cancer surgery. Eur J Cardio-thorac Surg. 2011;40(6):1432-8.
18. Hays JT, Croghan IT, Baker CL, et al. Changes in health-related quality of life with smoking cession treatment. Eur J Public Health. 2012;22(2):224-9.
19. Tammemagi CM, Neslund-Dudas C, Simoff M, et al. Lung carcinoma symptoms-an independent predictor of survival and important mediator of African-American disparity in survival. Cancer. 2004;101(7):1655-63.
20. Han KT, Park EC, Kim JH, et al. Is marital status associated with quality of life? Health Qual Life Outcomes. 2014;12:109.
21. Jatoi A, Novotny P, Cassivi S, et al. Does marital status impact survival and quality of life in patients with non-small cell lung cancer? Observations from the Mayo clinic lung cancer cohort. Oncologist. 2007;12(12):1456-63.
22. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40(5):373-83.
23. Wahlgren T, Levitt S, Kowalski J, et al. Use of the Charlson combined comorbidity index to predict prostradiotherapy quality of life for prostate cancer patients. Int J Radiat Oncol Biol Phys. 2011;81(4):997-1004.
Received: July 26, 2021;
Accepted: August 10, 2021;
Published: August 12, 2021.
To cite this article : Fukai R, Nishida T, Igarashi Y, et al. Perioperative Progress and Predictor of the Postoperative Mental Quality of Life for Lung Cancer Patients. British Journal of Cancer Research. 2021; 4:2.
©2021 Fukai R, et al.
