Research article / Open Access
DOI: 10.31488/bjcr.190
PET Scans for Locally Advanced Breast Cancer and Diagnostic MRI to Determine the Extent of Operation and Radiotherapy (PET LABRADOR): TROG 12.02
Kirsten Gadsby1, Tracy Pearl-Larsson1, Val Gebski1,2, Catherine Bone Saunders3, Tim Wang1, 4, Michelle Grogan5, Jennifer Harvey6, Martin Borg7, Mandy Taylor8, Scott Babington9, Rina Hui12,13, Frances Boyle 13,14, Owen Ung15,16, Shih-Chang Wang10,13, Christopher Kelly1, Drew Latty1, Jonathan Sykes1, Emily Flower1, Rosemary Balleine17, Madeleine King13, Eric Haul13,18, Peter Graham19, Raghwa Sharma20, Nirmala Pathmanathan4, Sally Lord2, Carol Whiteside21, Susan Grayson10, Sheryl Foster10,11, Verity Ahern1
1. Department of Radiation Oncology, Crown Princess Mary Cancer Centre, Westmead Hospital, Sydney, NSW, Australia
2. National Health and Medical Research Council Clinical Trials Centre, Sydney, NSW, Australia
3. Department of Nuclear Medicine, Westmead Hospital, Sydney, NSW, Australia
4. Westmead Breast Cancer Institute, Westmead Hospital, Sydney, NSW, Australia
5. Department of Radiation Oncology, Royal Brisbane and Women’s Hospital, Brisbane, QLD, Australia
6. Department of Radiation Oncology, Princess Alexandra Hospital, Brisbane, QLD, Australia
7. Adelaide Radiotherapy Centre, Adelaide, SA, Australia
8. Department of Radiation Oncology, Sir Charles Gairdner Hospital, Perth, WA, Australia
9. Christchurch Hospital, Christchurch, New Zealand
10. Department of Radiology, Westmead Hospital, Sydney, NSW, Australia
11. Faculty of Medicine and Health, University of Sydney, Sydney, NSW. Australia
12. Department of Medical Oncology, Crown Princess Mary Cancer Centre, Westmead Hospital, Sydney, NSW, Australia
13. University of Sydney, Sydney, NSW, Australia
14. Patricia Ritchie Centre for Cancer Care and Research, Mater Hospital, Sydney, NSW, Australia
15. University of Queensland, Brisbane, QLD, Australia
16. Breast and Endocrine Surgery Unit, Royal Brisbane and Women’s Hospital, Brisbane, QLD, Australia
17. Children's Medical Research Institute, Faculty of Medicine and Health, The University of Sydney, Sydney, NSW, Australia
18. Department of Radiation Oncology, Blacktown Cancer & Haematology Centre, Blacktown Hospital, Sydney, NSW, Australia
19. Department of Radiation Oncology, St George Hospital, Sydney, NSW, Australia
20. Anatomical Pathology, NSW Health Pathology, NSW, Australia
21. Trans-Tasman Radiation Oncology Group
*Corresponding author:Kirsten Gadsby, Department of Radiation Oncology, Crown Princess Mary Cancer Centre, Westmead Hospital, Sydney, NSW, Australia
Abstract
Background: Some patients with stages IIB (T2N0M0) or III non-inflammatory (locally advanced) breast cancer (LABC) may become suitable candidates for breast conservation surgery (BCS) rather than mastectomy following primary systemic treatment (PST) (i.e., chemotherapy +/- targeted therapy, trastuzumab). A more accurate assessment of residual disease after PST may be achieved by breast magnetic resonance imaging (MRI) and positron emission tomography-computed tomography (PET-CT) scans compared to clinical examination, ultrasound, and mammography, thereby better identifying patients in whom mastectomy can be spared. This study examines the feasibility of such an approach. Methods: Women diagnosed with LABC and suitable to receive PST, surgery, and adjuvant radiotherapy are eligible for this prospective single-arm phase II study. The objectives are to ascertain the feasibility and diagnostic accuracy of a policy of MRI and PET-CT directed management in this patient cohort. For 70 women to undergo BCS, it is estimated that 220 women will need to be enrolled. Breast MRIs and PET-CT scans are performed at baseline, prior to cycle 3 of PST and pre-surgery. PST is prescribed per institutional practices and must include trastuzumab for tumours over-expressing HER2. If disease remains inoperable after PST, patients receive pre-operative radiation treatment and if the disease is operable post PST, radiation is given post-operatively. Discussion:This study explores a management pathway for LABC patients that integrates MRI and PET-CT scans in a uniform and strategic manner. The aim is to identify which women will become candidates for BCS without compromising local control or disease-free survival. Correlation between the central review of biopsy and surgical pathology to the MRI and PET-CT scans will be performed to determine the accuracy, sensitivity, and specificity of the imaging assessments. The correlative results will contribute to predicting which LABC patients may become suitable for BCS through such an imaging schedule. Furthermore, early indication of non‐response through imaging may prevent a patient receiving further ineffective systemic therapy with unnecessary toxicity and cost.
Background
Locally Advanced Breast Cancer (LABC) accounts for approximately 10% of new breast cancer presentations in Australia and New Zealand [1,2] . LABC is not easily operable initially, due to its size, involvement of skin or chest wall, or bulky regional nodes. Instead of immediate surgery, primary systemic therapy (PST), typically combination chemotherapy and targeted therapies (e.g., Trastuzumab) to render surgery feasible is common [3,4].
Studies of the management of LABC have focused on rates of pathological complete response as the main outcome of interest when assessing the efficacy of new primary systemic therapy agents [5-7]. There is less focus on surgical approaches following PST. Across Australia and New Zealand clinical practice has varied for women with LABC, particularly whether breast conservation surgery (BCS) is offered as an alternative to mastectomy, and which imaging modalities are employed to aid decision making for surgery.
Traditionally, tumour response is assessed by clinical examination and serial ultrasound although this approach can underestimate non-invasive cancer components such as ductal carcinoma in situ (DCIS). To mitigate the limitations of serial ultrasound, mastectomy is often recommended following PST. However, this may lead to unnecessary disfigurement, reconstructive costs, and morbidity.
The introduction of breast MRI and PET-CT scans is variable across Australian centres for management of LABC patients, particularly, the time points of a patient’s journey these are performed [8-11]. While breast MRI may not be accessible for many women in Australia depending on where they live, Medicare rebates for breast MRI became available in 2019 for women at diagnosis when other imaging is inconclusive, or for pre-surgical planning where there is discrepancy between clinical assessment and conventional imaging assessment [12]. Whole body PET scans also became available for Australian women for the staging of stage III LABC in 2019, although not for pre-surgical planning [13]. 18F- fluorodeoxyglucose (18F-FDG) is the most widely used radiotracer for PET scans in breast cancer and is routinely available in sites accredited for performing PET-CT studies by the Australian and New Zealand Association of Physicians in Nuclear Medicine (ANZAPNM) Practice Accreditation Program, and was therefore chosen as the radiotracer for this study [14]. Accurate assessment of the extent of disease by imaging before, during and after PST is necessary to ensure correct decisions are made about the extent of surgery undertaken (BCS or mastectomy) and radiation treatment (RT) target volumes.
Breast MRI assesses initial disease extent and its response to PST more accurately than physical examination, mammography/ultrasound [15-18]. However, a meta-analysis of surgical outcomes following pre-operative breast MRI for women with early-stage disease showed that MRI significantly increased mastectomy rates [19]. More recent studies report that breast MRI may over-estimate or under-estimate residual disease after PST [20] indicating that the use of MRI in LABC after PST still warrants investigation.
The main benefit of PET‐CT scans may be in their ability to detect distant disease with greater sensitivity than whole body bone scan and CT scans [21], although data is emerging regarding its use to assess disease extent in the breast and regional nodes, and to assess response to PST [22].
This study proposes a standard management pathway for LABC patients which integrates MRI and PET-CT scans in a uniform and strategic manner aiming to identify patients who can avoid mastectomy without jeopardising loco-regional control of disease. The primary objective of the study is to demonstrate a local recurrence (LR) rate of ≤ 20% at three years in patients who undergo BCS based on MRI and PET-CT scans and histopathology, noting that LR ≤ 10% is expected. LR rates after BCS for women with LABC range from 5% to 19% at five years [23-25]. Many factors influence local control, with higher rates of LR noted for patients with clinically involved axillary nodes and oestrogen receptor negative tumours [26] in one study and higher AJCC stage, negative progesterone receptor status and number of positive axillary nodes [27] in another. One retrospective study of women with LABC under the age of 45 years did not find a higher rate of LR for those undergoing BCS compared to mastectomy, although LR was higher with higher post PST nodal stage and AJCC stage [28].
Methods
This study is a single arm phase 2 study for patients with diagnosed LABC suitable for chemotherapy, surgical resection, and RT. The study is sponsored by TROG Cancer Research and has been granted ethics approval through the Western Sydney Local Health District (HREC:AU RED/13/WMEAD/464).
The primary endpoint of this study is to measure the local recurrence rates for all patients at 3 years. There are many secondary endpoints which are listed as follows:
● Presence of disease in the breast and nodes on pre‐treatment biopsies and pathology of definitive surgical specimen (or post‐PST biopsies in the event that disease remains inoperable after PST).
● Response on biopsies performed at Week 6 after start of PST (prior to cycle 3) and Week 3‐5 from last PST, and changes in MRI and PET‐CT scan tumour / node size and characteristics.
● Sites of distant disease as measured by PET‐CT compared with bone scan and CT scan head, chest, abdomen and pelvis.
● Presence or absence of disease in sentinel node(s).
● Disease free survival
● Sites of local and regional recurrence
● Quality of Life for all women (including body image).
● The cosmetic outcome for all women (objective and subjective, patient and clinician rated).
● Presence or absence of lymphoedema
● Patients’, medical oncologist’s, and surgeons’ decisions about treatment.
● The cost of MRI and PET‐CT and additional costs associated with these procedures.
● The cost of BCS compared to mastectomy.
● A bio‐bank of tumour and serum samples.
Patient will enter a two-stage registration process prior to entering PET LABRADOR, trial registration and then treatment registration. The trial registration inclusion and exclusion criteria are summarised in Table 1.
Table 1.PET LABRADOR Eligibility Criteria
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Females, ≥ 18 | Pregnant or lactating |
| Cytological +/‐ Histological confirmation of breast cancer | Unwilling to have photographs taken of the area from the mid‐ neck to navel |
| Clinical Stage IIB (T3N0M0) ‐ III (non‐inflammatory) unilateral breast cancer | Implanted medical or electronic devices deemed a contraindication to performing a breast MRI |
| Adequate haematological, renal, and hepatic function | Clinical evidence of bilateral breast cancer |
| Suitable for radical treatment employing chemotherapy, surgery, and radiation therapy | Previous RT to the area to be treated |
| ECOG performance 0‐1 | Previous chemotherapy or hormone therapy for this cancer |
| Life expectancy > 36 months | Previous surgery to the ipsilateral breast or nodes |
| Use of adequate contraception for participants capable of childbearing | Previous contralateral breast cancer |
| Available for follow up | Prior diagnosis of cancer with subsequent evidence of disease recurrence or clinical expectation of recurrence is greater than 10% within 5 years of current diagnosis except for successfully treated basal cell or squamous cell skin carcinoma or carcinoma in situ of the cervix |
| Patients with clinical evidence of metastatic disease |
Patients meeting the Trial Registration criteria patients will be registered to receive a baseline echocardiogram, FDG PET-CT, and a breast MRI scan. Following these investigations, patients will be excluded from Treatment Registration if it is found there are ≥ 4 sites of metastatic disease or inadequate cardiac function (LVEF ≤50%). Patients found to have bilateral breast cancer, who had clinically unilateral breast cancer at the time of trial registration will not be excluded. Patients who have sufficient cardiac function (LVEF >50%) for chemotherapy and up to 3 sites of distant metastases on FDG PET-CT scans can be registered on PET LABRADOR and will proceed with data collection and ongoing imaging as per protocol. The study schema is summarised in Figure 1.
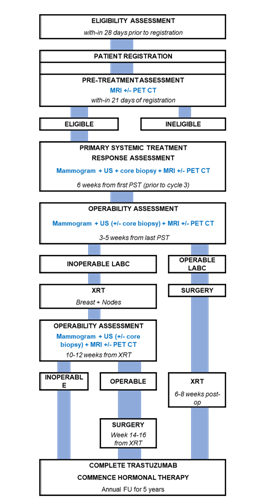
Figure 1:PET LABRADOR Schema
Treatment
All patients receive neo-adjuvant chemotherapy according to a recruiting centres standard, and targeted therapies if indicated (e.g., Trastuzumab). Registration onto additional clinical trials with alternative PST schedules will be permitted. Patients should receive a minimum of 6 weeks (or 2 cycles) of an anthracycline-based chemotherapy schedule, and a taxane for a minimum of 6 weeks (or 2 cycles). Hormonal therapy as an alternative to neo-adjuvant chemotherapy is not encouraged on this study unless a patient is enrolled on a separate study examining this question.
All PST should be given and completed before loco-regional treatment (i.e., surgery or RT). A minimum of 18 weeks and maximum of 26 weeks of PST is to be prescribed.
Patients will undergo breast MRI, PET-CT, ipsilateral mammogram, and core biopsy prior to cycle 3 of PST (approximately 6 weeks from first PST). A change in PST plan should be considered for patients whose imaging shows signs of progressive disease.
Following completion of PST patients will undergo repeat breast imaging which will include breast MRI, PET-CT scan and ipsilateral mammogram 3-5 weeks post completion. Patients deemed operable following PST will undergo surgery 5-7 week post the last day of PST. Each patient’s multidisciplinary team in consultation with the patient will determine the appropriate surgery based on imaging and physical assessment of response.
RT will be delivered 6-8 weeks from the date of last surgery, when a patient was operable post PST. For patients found to be inoperable following PST, RT will begin 5-7 weeks after last PST. RT will be given daily 9-10 times per fortnight, as per local practice. With interruptions, such as machine breakdown, treatment side-effects, service days, or public holidays managed as per local procedure.
The first five (5) patients enrolled in the study from each participating centre will undergo post RT quality assurance (QA) review. This review aims to ensure that the RT plan is developed according to the protocol. If the initial review results are acceptable, the trial site will proceed to a sampled review of 1-in-5 patients.
Women with oestrogen sensitive tumour will receive hormonal therapy for a minimum of 5 years, commencing 2 weeks after the last radiation treatment.
Imaging Protocols
Imaging protocols are summarised as follows:
MRI Protocol
A dedicated breast coil will be used on a 1.5T or 3T MRI scanner.
Pre-contrast scans
● Axial T2-W FSE
● Axial STIR
● Axial Diffusion-weighted imaging (DWI) whenever available
● Axial 3D T1-W gradient echo without fat suppression
● Axial 3D T1-W gradient echo with fat suppression (pre)
Post-contrast scans
Multi-phase Axial 3D T1-W gradient echo with fat suppression (same as pre contrast phase). Each phase to run for 62.5 sec with a 25 sec pause between the pre-contrast phase (1st phase) and the first post contrast phase (2nd phase), with a total of 5 post phases to be completed.
A bolus injection of a macrocyclic gadolinium-based contrast agent (0.1mmol/kg) is injected during the dynamic acquisition of the above sequence.
PET/CT Protocol
Imaging performed at ANZAPNM accredited sites by accredited staff. 18-F FDG dose of 5.3MBq/kg for PET scanner without time-of-flight capability. 18-F FDG dose reduced for high-count rate PET/CT scanners with time-of-flight capability including dose correction for body mass index.
● A cannula with a three-way tap should be inserted into a vein on the contralateral side to the breast tumour after standard preparation.
● Patients should be scanned in a standard position and replicated for sequential scans.
● An uptake period of 60min +/- 15min will be used for the baseline scan. Subsequent scans will use the same uptake time as baseline +/- 5min and performed on the same PET/CT scanner.
● Imaging acquired from vertex to proximal femora.
● A low dose CT scan without breath hold will be acquired first for attenuation correction.
● PET acquisition parameters and length of time for each bed position must remain stable for sequential scans in an individual patient (based on camera manufacturers recommendations/EANM guidelines).
● The first PET bed position should start at the vertex extending caudally.
● Image reconstruction parameter standardised and replicated for sequential scans.
Assessments
Patients will have chemotherapy, surgical and RT related acute and late toxicities reported using the NCI Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 [29].
Cosmetic breast photos will be taken throughout the trial to be used as an objective assessment of breast cosmesis. Photos will be taken pre-treatment, at operability review, post-op, post RT and then annually. The patient’s skin will be marked at the sternal notch and at 25cm inferior to the sternal notch at the midline. Additional photos will be taken where the patient has any palpable breast tumour or lymph nodes. All palpable disease will be outlined on the patient. Photos of outlined disease will be taken to visualise all markings, one photo with a ruler against the primary tumour will also be taken to enable verification of the size.
Two subjective cosmetic rating measures will be used. The clinician rated EORTC Cosmetic Assessment Rating System, and the patient rated Breast Cancer Treatment Outcome Scale (BCTOS) [30].
Cosmetic based assessments (EORTC Cosmetic Assessment Rating, Photos, and BCTOS PRO) will be discontinued post-operatively for patients who undergo mastectomy.
Patient Reported Outcomes (PROs) will be assessed using the EORTC QLQ-C30, EORTC QLQ-BR23, and EORTC EQ-5D-5L.
Statistical Methods
The study design is a single-arm prospective study. It is anticipated that 30% of patient will undergo BCS. Rates of BCS vary in the literature and 30% was set as a realistic percentage [31,32]. To detect, with 90% confidence (one-tailed) and 80% power, a local recurrence rate of less than 20% at three years, 70 patients with BCS will need to be enrolled. To achieve this sample size, 220 patients overall will need to be registered onto the trial. An interim analysis will be performed once 28 patients undergoing breast conservation surgery have achieved three years of follow-up. If five or more patients have experienced a local recurrence consideration will be given to stopping the study.
The primary endpoint will be reported as the proportion of patients who undergo BCS who have experienced a local recurrence, together with the 90% and 95% confidence interval for this proportion.
Discussion
This trial provides a standardised management pathway for women with LABC with an imaging protocol that aims to select the most appropriate surgical management (BCS or mastectomy) and the most appropriate RT volumes for individual patients based on their response to PST. Management varies for this stage of disease and the protocol offers a way of bringing together and improving the performance of a multi-disciplinary team of clinicians, pathologists, radiologists, and nuclear medicine specialists.
A small number of patients may benefit from study participation through the identification of distant disease that would dictate a different treatment plan. The major benefit of the protocol will be that if the imaging schedule proves to be an accurate predictor of disease response to PST for women with LABC, ineffective PST may be avoided, and individual patients offered a change to a potentially more effective PST regimen. A recently published randomised trial of PET-CT compared with conventional staging imaging for 369 women with TNM stage III or IIb breast cancer reported that more women were upstaged to metastatic disease and treatment was altered more often based on PET-CT findings [33]. Imaging might also be able to predict early in the treatment programme which patients will become BCS candidates and those who may still require a mastectomy.
The standardised imaging protocols for breast MRI and PET-CT allow the opportunity to build expertise in these modalities and will generate evidence to determine the added value and potential for these investigations in the management of LABC.
Several Australian and New Zealand studies have investigated the role of MRI in breast cancer management. The PROSPECT trial prospectively recruited 443 women with clinical T1N0 non-triple negative breast cancer to investigate whether pre-operative breast MRI and pathology findings could identify women who could avoid breast RT [34]. Women underwent BCS and if pT1No or N1mi, avoided RT. The ipsilateral invasive breast cancer recurrence rate at 5 years for this group of women was 1%. The ANZ 1401 ELIMINATE randomised trial of neoadjuvant chemotherapy with or without concurrent aromatase inhibitor to downstage oestrogen receptor positive Stage 2 or 3 breast cancer employed pre- and post-treatment breast MRI to determine the overall objective radiological response rate [9]. The primary endpoint was proportion of pathologic stage 0 or 1A at surgery and no information has been published regarding radiological response rate. Chan et al reported a study of 50 women with LABC who underwent PST using six cycles of chemotherapy (docetaxel, doxorubicin and cyclophosphamide) and incorporating PET-CT and breast MRI to assess accuracy of prediction of response [35]. This study found that MRI was the most accurate way of assessing for residual disease after PST and it was suggested that MRI could be used to select patients for BCS. The BCT 2001: Breast MRI Evaluation is an observational study that aims to find out the best way to use breast MRI for women with early breast cancer and if it will improve treatment options and patient outcomes for these women [11]. The results from this study are not yet reported. The investigation of MRI in PET LABRADOR remains relevant.
Since the PET LABRADOR protocol was developed, the field of radiomics has emerged and the use of MRI and PET-CT as a biomarker for response to PST is an area of interest [36-38]. There is now interest in developing a machine learning model that classifies response to PST based on MRI or PET-CT features rather than biopsy, and that will be more sensitive to detecting pathologic complete response (pCR) than current experience [39]. There is now early data supporting de-escalation of systemic therapy based on early PET-CT metabolic response for certain molecular sub-groups of breast cancer [40]. PET LABADOR will provide a unique prospective imaging data set pre-, during and post-PST, matched with biopsies during PST and histopathology after PST that will be sufficiently large to develop to develop a radiomics model for the prediction of pCR in LABC treated by PST, to validate other models, or to combine with other models to improve them. Local expertise exists in radiomics and medical image data augmentation techniques for deep learning applications [41,42]. Post the development of this protocol, the COVID pandemic is projected to have an impact on recruitment to the study.
Skin sparing mastectomy and oncoplastic techniques to extend the opportunity for breast conservation surgery are now more commonly available to women who have undergone PST for LABC [43,44]. However, the role of these techniques for LABC remains unclear [45] and the aims of PET LABRADOR remain current.
Abbreviations
ANZCTR: Australian New Zealand Clinical Trials Registry, BCS: Breast Conservation Surgery, BCTOS: Breast Cancer Treatment Outcome Scale, CT: Computed Tomography, CTCAE: Common Terminology Criteria for Adverse Events, DCIS: Ductal Carcinoma in-situ, DWI: Diffusion-weighted imaging, ECOG: Eastern Cooperative Oncology Group, EORTC: European Organisation for Research and Treatment of Cancer, FDG: Fludeoxyglucose F18, HER2: Human Epidermal Growth Factor Receptor 2, LABC: Locally Advanced Breast Cancer, LR: Local Recurrence, LVEF: Left Ventricular Ejection Fraction, MRI: Magnetic Resonance Imaging, pCR: Pathological Complete Response, PET-CT: Positron Emission Tomography-Computed Tomography, PRO: Patient Reported Outcome, PST: Primary Systemic Treatment, QA: Quality Assurance, RT: Radiation Treatment, STIR: Short Tau Inversion Recovery, TROG: Trans-Tasman Radiation Oncology Group, US: Ultrasound
Declarations
Trial registration
This trial was registered on ANZCTR (reg number: ACTRN12613000253707) on 4th Mar 2013.
Ethics approval
This trial (ACTRN12613000253707) has received ethical approval from the Western Sydney Local Health District Human Research Ethics Committee.
Competing interests
The authors declare no conflict of interest.
Funding
Funding was received from the Sydney West Radiation Oncology Network and Western Sydney Local Health District Nuclear Medicine provided in-kind PET-CT scans.
Authors' contributions
All author contributed to the development of the protocol, reviewed, and commented on the manuscript. KG, VG, VA were major contributors in writing the manuscript.
Acknowledgements
We would like to acknowledge the Trans-Tasman Radiation Oncology Group (TROG) for supporting trial.
References
1. Health AIo, Welfare. Cancer in Australia 2010: an overview. Canberra: AIHW; 2010. Health AIo, Welfare. Cancer data in Australia. Canberra: AIHW; 2022.
2. Tryfonidis K, Senkus E, Cardoso MJ, Cardoso F. Management of locally advanced breast cancer—perspectives and future directions. Nature Reviews Clinical Oncol. 2015;12(3):147-62.
3. Early and locally advanced breast cancer: diagnosis and management London: National Institute for Health and Care Excellence (NICE)14 Jun 2023 [Available from: https://www.ncbi.nlm.nih.gov/books/NBK519155/.
4. Gianni L, Eiermann W, Semiglazov V, Manikhas A, Lluch A, Tjulandin S, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010;375(9712):377-84.
5. Gianni L, Pienkowski T, Im YH, Roman L, Tseng LM, Liu MC, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(1):25-32.
6. Untch M, Rezai M, Loibl S, Fasching PA, Huober J, Tesch H, et al. Neoadjuvant treatment with trastuzumab in HER2-positive breast cancer: results from the GeparQuattro study. J Clin Oncol. 2010;28(12):2024-31.
7. Chan A, Willsher PC, Hastrich DJ, Anderson J, Barham T, Latham B, et al. Preoperative taxane-based chemotherapy in a standardized protocol for locally advanced breast cancer. Asia Pac J Clin Oncol. 2012;8(1):62-70.
8. Murray N, Francis P, Zdenkowski N, Wilcken N, Boyle F, Gebski V, et al. Randomized trial of neoadjuvant chemotherapy with or without concurrent aromatase inhibitor therapy to downstage ER+ ve breast cancer: Breast Cancer Trials Group ANZ 1401 ELIMINATE trial. Annals of Oncol. 2022;33:S164-S5.
9. Newman LA. Role of preoperative MRI in the management of newly diagnosed breast cancer patients. Journal of the American College of Surgeons. 2020;230(3):331-9.
10. An observational study to see if a breast Magnetic Resonance Imaging (MRI) of women recently diagnosed with breast cancer impacts on their treatment planning and cancer outcomes (BCT 2001) [updated 19 Aug 2022. Available from: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=379246.
11. Breast cancer MRI added to Medicare [press release]. 29 Oct 2019 2019.
12. New Medicare rebates for PET scans for breast cancer [press release]. 01 Nov 2019 2019.
13. Balma M, Liberini V, Racca M, Laudicella R, Bauckneht M, Buschiazzo A, et al. Non-conventional and Investigational PET Radiotracers for Breast Cancer: A Systematic Review. Front Med (Lausanne). 2022;9:881551.
14. Croshaw R, Shapiro-Wright H, Svensson E, Erb K, Julian T. Accuracy of Clinical Examination, Digital Mammogram, Ultrasound, and MRI in Determining Postneoadjuvant Pathologic Tumor Response in Operable Breast Cancer Patients. Annals of Surgical Oncol. 2011;18(11):3160.
15. Julius T, Kemp SE, Kneeshaw PJ, Chaturvedi A, Drew PJ, Turnbull LW. MRI and conservative treatment of locally advanced breast cancer. Eur J Surg Oncol. 2005;31(10):1129-34.
16. Londero V, Bazzocchi M, Del Frate C, Puglisi F, Di Loreto C, Francescutti G, et al. Locally advanced breast cancer: comparison of mammography, sonography and MR imaging in evaluation of residual disease in women receiving neoadjuvant chemotherapy. Eur Radiol. 2004;14(8):1371-9.
17. McLaughlin R, Hylton N. MRI in breast cancer therapy monitoring. NMR Biomed. 2011;24(6):712-20.
18. Houssami N, Turner R, Morrow M. Preoperative magnetic resonance imaging in breast cancer: meta-analysis of surgical outcomes. Ann Surg. 2013;257(2):249-55.
19. Reig B, Lewin AA, Du L, Heacock L, Toth HK, Heller SL, et al. Breast MRI for Evaluation of Response to Neoadjuvant Therapy. Radiographics. 2021;41(3):665-79.
20. Whitman GJ, Strom EA. Workup and staging of locally advanced breast cancer. Semin Radiat Oncol. 2009;19(4):211-21.
21. Hadebe B, Harry L, Ebrahim T, Pillay V, Vorster M. The Role of PET/CT in Breast Cancer. Diagnostics. 2023;13(4):597.
22. Chen AM, Meric-Bernstam F, Hunt KK, Thames HD, Oswald MJ, Outlaw ED, et al. Breast conservation after neoadjuvant chemotherapy: the MD Anderson cancer center experience. J Clin Oncol. 2004;22(12):2303-12.
23. Meyers MO, Klauber-Demore N, Ollila DW, Dees EC, Calvo BF, Moore DT, et al. Locoregional control in locally advanced breast cancer using neoadjuvant chemotherapy followed by breast conservation. Journal of Clinical Oncology. 2008;26(15_suppl):539-.
24. Zhou X, Li Y. Local Recurrence after Breast-Conserving Surgery and Mastectomy Following Neoadjuvant Chemotherapy for Locally Advanced Breast Cancer - a Meta-Analysis. Breast Care (Basel). 2016;11(5):345-51.
25. Chou H-H, Chung W-S, Ding R-Y, Kuo W-L, Yu C-C, Tsai H-P, et al. Factors affecting locoregional recurrence in breast cancer patients undergoing surgery following neoadjuvant treatment. BMC Surgery. 2021;21(1):160.
26. Levy A, Borget I, Bahri M, Arnedos M, Rivin E, Vielh P, et al. Loco-regional control after neo-adjuvant chemotherapy and conservative treatment for locally advanced breast cancer patients. Breast J. 2014;20(4):381-7.
27. Sweeting RS, Klauber-Demore N, Meyers MO, Deal AM, Burrows EM, Drobish AA, et al. Young women with locally advanced breast cancer who achieve breast conservation after neoadjuvant chemotherapy have a low local recurrence rate. Am Surg. 2011;77(7):850-5.
28. Common Terminology Criteria for Adverse Events (CTCAE) v4.0 2010 [updated 14 Jun 2010. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_40.
29. Stanton AL, Krishnan L, Collins CA. Form or function? Part 1. Subjective cosmetic and functional correlates of quality of life in women treated with breast-conserving surgical procedures and radiotherapy. Cancer. 2001;91(12):2273-81.
30. Nobrega GB, Mota BS, de Freitas GB, Maesaka JY, Mota RMS, Goncalves R, et al. Locally advanced breast cancer: breast-conserving surgery and other factors linked to overall survival after neoadjuvant treatment. Front Oncol. 2023;13:1293288.
31. Sun Y, Liao M, He L, Zhu C. Comparison of breast-conserving surgery with mastectomy in locally advanced breast cancer after good response to neoadjuvant chemotherapy: A PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore). 2017;96(43):e8367.
32. Dayes IS, Metser U, Hodgson N, Parpia S, Eisen AF, George R, et al. Impact of 18F-Labeled Fluorodeoxyglucose Positron Emission Tomography-Computed Tomography Versus Conventional Staging in Patients With Locally Advanced Breast Cancer. J Clin Oncol. 2023;41(23):3909-16.
33. Mann GB, Skandarajah AR, Zdenkowski N, Hughes J, Park A, Petrie D, et al. Postoperative radiotherapy omission in selected patients with early breast cancer following preoperative breast MRI (PROSPECT): primary results of a prospective two-arm study. Lancet. 2023.
34. Chan A, Hastrich D, Ingram D, Anderson J, Barham T, Schaaf Avd, et al. Final results of XRP6976D: Preoperative TAC (docetaxel, doxorubicin, cyclophsophamide) in conjunction with the development of a standard protocol for the management of locally advanced breast cancer. J Clin Oncol. 2008;26(15_suppl):628-.
35. Ha S, Park S, Bang J-I, Kim E-K, Lee H-Y. Metabolic Radiomics for Pretreatment 18F-FDG PET/CT to Characterize Locally Advanced Breast Cancer: Histopathologic Characteristics, Response to Neoadjuvant Chemotherapy, and Prognosis. Scientific Reports. 2017;7(1):1556.
36. Sharma U, Agarwal K, Sah RG, Parshad R, Seenu V, Mathur S, et al. Can Multi-Parametric MR Based Approach Improve the Predictive Value of Pathological and Clinical Therapeutic Response in Breast Cancer Patients? Frontiers in Oncol. 2018;8.
37. Sutton EJ, Onishi N, Fehr DA, Dashevsky BZ, Sadinski M, Pinker K, et al. A machine learning model that classifies breast cancer pathologic complete response on MRI post-neoadjuvant chemotherapy. Breast Cancer Res. 2020;22(1):57.
38. Gu Y-L, Pan S-M, Ren J, Yang Z-X, Jiang G-Q. Role of Magnetic Resonance Imaging in Detection of Pathologic Complete Remission in Breast Cancer Patients Treated With Neoadjuvant Chemotherapy: A Meta-analysis. Clinical Breast Cancer. 2017;17(4):245-55.
39. Gebhart G. 18F-FDG PET “Metabolic Response” to Neoadjuvant Systemic Therapy for Breast Cancer: Quo Vadis? J Nuclear Med. 2023;64(11):1697-8.
40. Sun Y, Reynolds HM, Parameswaran B, Wraith D, Finnegan ME, Williams S, et al. Multiparametric MRI and radiomics in prostate cancer: a review. Australas Phys Eng Sci Med. 2019;42(1):3-25.
41. Chlap P, Min H, Vandenberg N, Dowling J, Holloway L, Haworth A. A review of medical image data augmentation techniques for deep learning applications. J Med Imaging Radiat Oncol. 2021;65(5):545-63.
42. Malya FU, Kadioglu H, Bektasoglu HK, Gucin Z, Yildiz S, Guzel M, et al. The role of PET and MRI in evaluating the feasibility of skin-sparing mastectomy following neoadjuvant therapy. J Int Med Res. 2018;46(2):626-36.
43. Sang Y, Zhou X, Chi W, Chen J, Yang B, Hao S, et al. Surgical options of the breast and clinical outcomes of breast cancer patients after neoadjuvant chemotherapy: A single-center retrospective study. Frontiers in Oncol. 2022;12.
44. van la Parra RFD, Clough KB, Thygesen HH, Levy E, Poulet B, Sarfati I, et al. Oncological Safety of Oncoplastic Level II Mammoplasties After Neoadjuvant Chemotherapy for Large Breast Cancers: A Matched-Cohort Analysis. Annals of Surgical Oncol. 2021;28(11):5920-8.
Received: February 12, 2024;
Accepted: March 04, 2024;
Published: March 06, 2024.
To cite this article : Gadsby K, Pearl-Larsso Tn, Gebski V, Saunders CB, Wang T, Grogan M, et al. PET Scans for Locally Advanced Breast Cancer and Diagnostic MRI to Determine the Extent of Operation and Radiotherapy (PET LABRADOR): TROG 12.02. British Journal of Cancer Research. 2024; 7(1): 656- 663. doi: 10.31488/bjcr.190.
© The Author(s) 2024.
