Research Article / Open Access
DOI: 10.31488/bjcr.158
Pilot Study of Frailty Evaluation and Treatment in Elderly Cancer Patients
Arroyo Salgado A.*1, Fernandez Fenoll S.*2, Campello Garcia E.*3, Fajardo Sebastian S.*4, Baron Saura J. *5, Asensio Martinez E.*6, Gallego Plazas J*7
1.ICO Institut Català d'Oncologia.
2.Elche Universitary General Hospital, Camí L Alzamara 11, 032013, Elche, Spain
3.R & D project management area FISABIO. Elche Universitary General Hospital, Camí L Alzamara 11, 032013, Elche, Spain
*Corresponding author:Arroyo Salgado A., Ph.D, ICO Institut Català d'Oncologia. Hospital Duran i Reynals, Avinguda de la Gran Via de l'Hospitalet, 199-203, 08908 Hospitalet de Llobregat, Barcelona (Spain).
Abstract
Introduction: Patients older than 70 years have different states of frailty, risk of toxicity and mortality. An integral geriatric evaluation (IGE) could contribute to determine a better treatment approach in these patients. Objectives: To investigate recommendation of treatment based on IGE. Evaluate the feasibility of the IGE, and finally to compare the IGE group with a cohort of patients who received cancer treatment according to medical criteria and without previous geriatric tests (historical group). Methods: Through an observational prospective cohort study, 32 patients older than 70 years with colorectal cancer stage III (CRC III), IV (CRC IV) and non-small cell lung cancer stage IV (NSCLC IV) were evaluated. IGE was performed to patients, including evaluation of frailty, risk of toxicity and mortality. With these results, a standard treatment, modified treatment or no chemotherapy treatment was recommended Patients were followed for six months after treatment decision. Results: Average age was 79.3 years and 71.9% were male. A different treatment from the standard was recommended for 70% of patients with a diagnosis of CRC III, 90% with CRC IV, and 91.6% patients with NSCLC IV. The median time for IGE was 36,5 minutes. Conclusion: The use of IGE in cancer patients older than 70 years is feasible and useful for treatment recommendation.
Keywords: integral geriatric evaluation, cancer, treatment recommendation, feasibility, risk of toxicity and mortality
Introduction
The aging of the population constitutes a great challenge for the planning and delivery of health services. Cancer disproportionately affects the elderly, and more than a third of them are diagnosed in those older than 70 years [1]. Approximately 60% of all cancers and 70% of deaths caused by cancer occur in people older than 65 years [2-4]. In Europe and Spain, colorectal and lung cancer are among the 3 most frequent cancers in incidence and mortality, with the mean age at diagnosis being 72 and 68 years respectively [5,6].
The recommendation of antineoplastic treatment in patients older than 70 years should depend on factors that may include spheres of biological status, functional status, social status, comorbidity, psychological status, cognitive and socioeconomic status [7]; However, there are no studies that take into account all the aforementioned factors to decide the approach to cancer treatments in these patients [8-10].
Current clinical practices often recommend treatments based on the judgment of the physician, which often includes clinical experience, associated with other general tests such as functional status (PS) and Karfnosky score (KS), without offering tests or evaluations that can determine this objectively and reproducibly [8-10]. This is because many of the treatments approved in these guidelines arise from clinical trials that also do not use any specific test for this older population, so they are generally not included or are underrepresented [9]. Knowing that tolerance to treatments, side effects and adherence to them is different in older patients [9], a comprehensive global assessment that is feasible in routine clinical practice, and that may be equally valid for the most common cancers, such as colon and lung, are essential in older adults [8-10].
A comprehensive geriatric assessment of the elderly patient, which contributes to an individualized treatment recommendation, could have consequences in terms of therapeutic efficacy, quality of life, healthcare costs and hospital admissions, with potential benefits for this group. patients and society [11].
The objectives of the pilot study were a) to determine a treatment recommendation based on IGE, b) to evaluate the viability of IGE and c) to compare the results of treatment based on the IGE, with a historical cohort of patients that reflect previous healthcare practice, analyzing the differences between both approaches.
Methods
Design and patients
A prospective and observational cohort study was proposed. The patients were included consecutively upon arrival at the Medical Oncology service and treated on an outpatient basis, including 32 patients (IGE group): 10 patients with stage III colorectal cancer (CRC III), 10 patients with stage IV colorectal cancer (CRC IV) and 10 patients diagnosed with stage IV non-small cell lung cancer (NSCLC IV). The patients were recruited at the Hospital Universitario de Elche (Spain) from June 2012 to February 2017. The inclusion criteria were age ≥70 years, confirmed histological diagnosis of CRC III, CRC IV and NSCLC IV, minimum life expectancy of 6 months and written informed consent. The patients were followed for six months. The study was presented and approved by the Ethics Committee on December 20, 2012. All people gave their informed consent before being included in the study.
Procedures
IGE was performed to patients, including different aspects of the biological age of the elderly patient: nutritional status (Mini nutritional assessment test), functional status (Test get up and go, katz test, lawton and brody test, Karfnosky index, perfomance status and fall test), comorbidity (Charlson test and ACE-27), cognitive status (Minimental test), psychological state (Yesavage test), and social status (MOS test), during the first consultation to the Oncology service Medical [12-20]. According to the test results, we proceeded to stablish treatment recommendation with standard chemotherapy, modified treatment or no active treatment (Table1).
Subsequently, patients were followed up over time, recording hospital admissions, changes in treatment dose, toxicity and death for 6 months after the IGE. Duration of each test used in the IGE was also measured in minutes, in order to determine the feasibility of IGE in clinical practice (Figure 1).
Parallel to the recruitment of patients, data from 30 medical records (historical group) of patients diagnosed with CRC III (10 patients), CRC IV (10 patients) and NSCLC IV (10 patients) were reviewed. In both groups demographic data of the patients were recorded, as well as the chemotherapy treatment schemes and doses used; toxicities, hospital admissions and death, in a period of 6 months after the start of the study were also recorded.
Our study defined fluoropyrimidine-based chemotherapy with oxaliplatin (CAPOX, FOLFOX) as a standard treatment in CRC III [21]. With regard to CRC IV, chemotherapy was defined as standard according to the scheme 5-fluoracil with oxaliplatin or irinotecan associated or not with anti-EGFR or anti-VEGF treatment [22]. In stage IV lung cancer, standard chemotherapy treatment included platinum chemotherapy combinations [23]. All antineoplastic treatment not considered standard was considered modified [21-25].
Treatment recommendation was based on the risk of mortality at 6 months, risk of toxicity and frailty collected in the IGE. The risk of mortality at 6 months, based on the test developed by Souberyan et al. determined a high or low mortality risk. The risk of toxicity was based on the test developed by Hurria et al. determined high or medium toxicity risk and fragility was performed using the test developed by Khöne et al. which described patients as fragile, non-fragile or intermediate [26-28]. With these results, a standard treatment, modified or non-antineoplastic, was recommended (Table 1).
Table 1. Treatment selection in IGE group
| Strategic of treatment | Frailty1 | Toxicity2: | Mortality3: | ||
|---|---|---|---|---|---|
| Standard Treatment: | non-frail | and → | low risk | and → | low risk |
| Modified Treatment: | medium | and → | low/medium risk | and → | low risk |
| Non- Treatment: | frail | and/or → | high risk | and/or → | high risk |
1:Frailty: frail, medium, non-frail, 2:Toxicity: low, medium or high, 3:Mortality: low or high.
Statistical analysis
The SPSS version 17.0 program was used, both for the 32 patients of the IGE group, and for the 30 medical records of the historical group. The statistical tools used were frequencies (percentages and proportions) for the description of the results. The Spearman and Tau-b Kendall tests were used to relate the treatment recommendation obtained with the risk of mortality and toxicity.
Additionally, the Monte Carlo, Chi square and Mann Whitney statistical tests were used to search for relationships between adverse effects of treatment, mortality, hospital admissions and treatment modifications, between the IGE group and the historical group.
Results
In the IGE group, 32 patients were evaluated: 10 patients with stage III colorectal cancer (CRC III), 10 patients with stage IV colorectal cancer (CRC IV), and 12 patients with a diagnosis of non-small cell lung cancer in stadium. IV (CPCNP IV). 23 (71.9%) men and 9 (28.1%) women, with a mean age at diagnosis of 79.3 years (range 71 to 85) (Table 2).
Table 2. Epidemiological data and results of tests in the IGE group
| Data of IGE | CRC III1 (n: 10) | CRC IV 2(n: 10) | NSCLC IV3 (n:12) |
|---|---|---|---|
| Medium of age (years) | 80 | 79,4 | 79,6 |
| Men/women (%) | 60/40 | 70/30 | 83/17 |
| Weight loss4 | 10 | 0 | 25 |
| ECOG 3-4 (%) | 10 | 10 | 58,3% |
| IMC (Kg/m2) | 28,5 | 28,7 | 24,6 |
| Frailty5 (%) | 80/20 | 80/20 | 25/75 |
| Risk of Mortality6(%) | 90/10 | 100/0 | 53/46 |
| Risk of Toxicity7 (%) | 20/80 | 10/90 | 50/50 |
1:CRC III: colorectal cancer stage III, 2:CRC IV: colorectal cancer stage IV, 3: NSCLC IV: non small cell lung cancer stage IV, 4: weight loss4 > 10% in 6 months (%) 5:Frailty: frail, medium, non-frail, 6:Toxicity**: low, medium or high, 7: Mortality: low or high.
According to the tests performed, the results of the fragility, mortality and toxicity tests were obtained. 6 (18%) of the entire sample were considered non-frail patients. 7 (21.9%) had a high risk of toxicity and 6 (18%) had a high risk of early mortality (Table 2).
Treatment recommendation
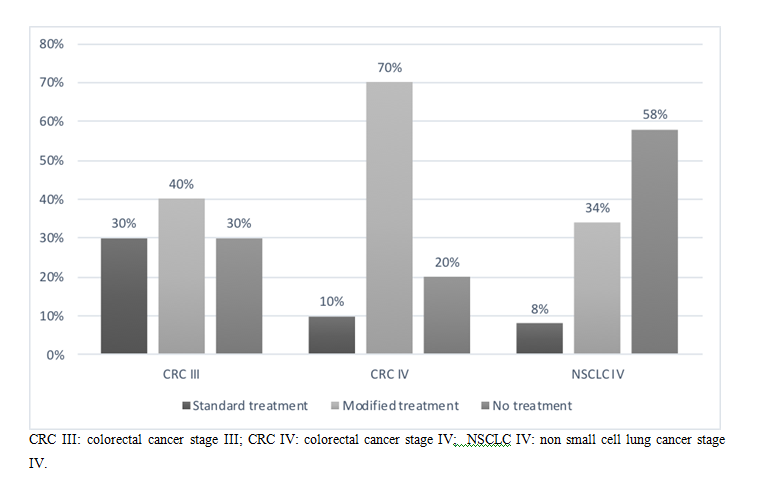
According to the results obtained, 7 (70%) of the patients with diagnosis of CRC III, 9 (90%) with CRC IV and 11 (91.6%) patients with NSCLC IV, had a treatment recommendation different from the standard (no treatment or modified treatment). 12 (37.5%) patients in the sample were advised not to receive treatment: 3 (30%) patients with CRC III, 2 (20%) CRC IV, and 7 (58%) NSCLC IV (Figure 2).
Figure 2. Treatment recommendation in IGE group
A correlation between treatment recommendation and adverse effects G3-4 (AEs) was evaluated using the Spearman and Tau-b Kendall analysis; this analysis showed an statistically relationship (p: 0.05) between the two variables in an inversely proportional direction (Spearman 0.034 and Tau-b Kendal 0.030) with a correlation coefficient according to Spearman -0.454 and Tau-b Kendall -0.473. This means that, based on this analysis, treatment recommendation different from standard correlates with lower toxicity.
We also observed an statistically significant inversely proportional correlation (p: 0.01) between treatment recommendation and mortality risk (Spearman 0.002 and Tau-b Kendall 0.001) with a correlation coefficient according to Spearman -0.54 and Tau-b Kendall -0.57. These results suggest that a lower risk of early mortality correlates with a modified treatment recommendation or no treatment.
Feasibility of the integral geriatric evaluation
The duration of the interview of the different tests and scales was quantified in minutes and performed to the 32 patients of the study; in addition, the total time spent in the different tests was quantified. The Charlson and ACE-27 tests were excluded since data from these evaluations were included in the clinical records.
It was observed that the MMSE-30 test was the one that occupied the longest time with an average of 8.34 minutes, followed by the MNA test with 7.54 minutes. The tests that required less time to complete were the Karnofsky index with 0.89 minutes and the stair climbing test with an average of 0.96 minutes. The total time of the tests evaluated in the 32 patients was 36.5 minutes on average, with a minimum value of 21 minutes and a maximum of 75 minutes.
Comparison of the treatment recommendation determined in the IGE group with the historical group
In reference to the treatment administered in the historical group, it was observed that 11 (40%) patients received a standard treatment, 10 (33%) patients had a modified treatment, and 9 (27%) patients did not receive treatment.
According to the primary tumor and stage we observed that in the historical group, 2 (20%) 4 (40%), and 3 (30%) patients with CRC III, CRC IV and NSCLC IV respectively, received a standard treatment. Likewise, 6 (60%), 2 (20%) and 5 (50%) of these patients did not receive treatment (Figure 3).
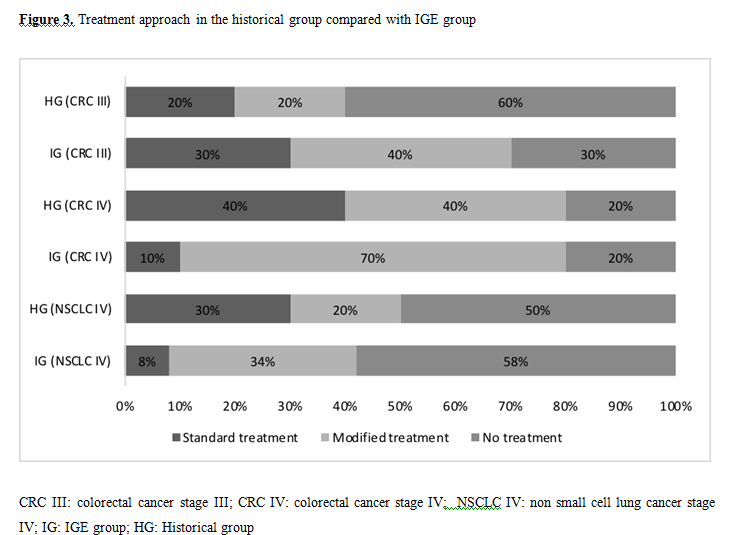
In relation to the secondary effects, grades 3-4, related to treatment between the IGE group and the historical group, we observed in CRC III (20% vs 30% respectively) in CRC IV (55 vs 40%) and in NSCLC IV (8 vs 20% respectively) In relation to mortality, we observed in CRC III (10% of the IGE group vs 20% of the historical group), CRC IV (20% vs 40%) and in NSCLC IV (76% vs 40% respectively).
When statistical tests (Monte Carlo, Chi-square, and Mann Whitney test) were used to look for relationships between treatment recommendation in both groups, treatment adverse effects, mortality, hospital admissions, and treatment modifications, no found statistically significant results with the tools used.
Discussion
In our study, we observed that the recommendation of treatment in patients, as a result of IGE, tend to be restrictive with respect to the literature reviewed [29-31], observing that 84.3% of the sample was recommended a treatment different from the standard and 37.5% of the evaluated patients were recommended not to receive a specific treatment; however, there were differences in the study results between primary tumor and stage.
In relation to CRC III we observed that 40% of the patients were recommended a modified treatment and 30% of them were not recommended any treatment. It is interesting to note that 80% of this group was categorized as perfomance status (PS) 0-1 and Karnofsky score (KS) greater than 80, in whom theoretically could receive a standard treatment. Possibly the fact of running a test that was endorsed for patients with advanced disease and not with adjuvant intention is an important factor to be taken into account. Despite all this, it seems that the recommendation groups of the study treatment allow to treat a larger group of patients than in the usual clinical practice where, according to historical series, approximately 50% of the elderly patients with CRC III do not receive adjuvant treatment [32,33].
With regard to patients with CRC IV, 80% of patients were recommended oncological treatment different from the standard and 20% of patients were recommended not to receive treatment. When looking at the PS and KS scale in this group of patients we see that 60% of these were considered as PS 0-1 and 90% with KS greater than 60, theoretically able to receive treatment. Currently the PS and KS tests are not the only factors to determine the composition of an oncological treatment. The location and number of metastases, the possibility of surgical resectability of the lesions and the functional consequences on the patient are important factors to be taken into account.
Regarding patients with NSCLC IV, the treatment recommendation, based on the geriatric evaluation of the study, tended to be more restrictive compared to the literature. We observed that 58% of patients were recommended not to receive treatment and 8% were recommended a standard treatment. Comparing it with the PS and KS scale, we observed that 16% were PS 0-1 and 67% had a KS greater than 60, in which some type of treatment could have been considered. These findings can also be explained by the difference between the results of the frailty, mortality and toxicity tests. In these tests, the results obtained in colon cancer III and IV were correlated with the literature obtained [26-28]; however, the data obtained for patients with NSCLC IV were different.
Regarding the risk of toxicity, a tendency is observed in our study to group patients with low risk. Probably the score for the instrumental activities of daily living, creatinine clearance, as it was not genitourinary or digestive pathology (having a higher risk score), influenced the final result. Regarding the risk of early mortality [26], we also observed clear differences between the digestive and pulmonary pathology of the study. This test is based on nutritional and functional tests, where our patients with lung cancer obtain worse results. It must be taken into account that Souberyan's analysis was designed in patients mainly with hematological (57%) and gastric (30%) tumors, so that probably the results in lung tumors, which present higher comorbidity, are associated with worse scores [26].
We observed a statistically significant result when correlating treatment recommendation with grade 3-4 adverse effects and mortality. As a result, the higher percentage of recommended standard treatment produced greater adverse effects and higher mortality. These results show that, in spite of selecting patients with different strategies, the toxicity caused by treatment is important with special impact in the advanced pulmonary pathology, and that the risk of mortality is one of the determining factors in the decision of treatment recommendation.
Regarding the feasibility of the IGE, an average duration of 36 minutes was determined. Considering the average duration of a medical consultation, the performance of this test requires an additional prior consultation. The benefits that could be obtained in the reduction of pharmacological expenses, reduction of hospital admissions, treatment of complications due to toxicity and planning of symptomatic domiciliary treatment, would justify the execution cost.
When comparing the results of the treatment recommendation of the IGE group with the historical group, we observed that, despite not obtaining statistically significant data, more patients with CRC III of the IGE group were treated with adjuvant chemotherapy and also, the patients of this group they presented fewer adverse effects to treatment with similar mortality. These data encourage the use of IGE in these patients in order to optimize adjuvant treatment.
The patients with CRC IV of the IGE group were treated in the same number as the historical group, but with a higher percentage of monotherapy treatment (70% vs 40%). These patients had a higher percentage of adverse effects (55 vs 40%), however they presented lower mortality (20% vs 40%), these data, even though it was not statistically significant, could suggest the potential utility of the IGE to avoid deaths related to oncological treatment and in determining the use of monotherapy treatment.
Regarding NSCLC IV, Patients in the IGE group had a higher proportion of monotherapy treatment (33 vs 20%), having fewer adverse effects (8 vs 20%), but higher mortality (76 vs 40%). Although these data were not significant according to our tools used, possibly the differences in the percentage of mortality in this group are due to PS 3-4 in both groups (58% in the IGE group compared to 10% in the historical group). A more homogeneous selection of the population of this pathology is essential to make recommendations using the IGE.
Probably the fact that these differences in the recommendation of treatment between the IGE group and the historical group were not reflected in the statistical analysis carried out may be due, among other factors, to the sample size and the heterogeneity of the tumor types, points that We strongly believe that they should be taken into account in future studies.
It could be concluded from the study that the geriatric assessment in these patients is feasible and could have a real impact on treatment recommendation, on the incidence of adverse effects derived from it, as well as on the early mortality of patients. A study including patients with other cancer types, with larger sample size is underway in our centre. It would be advisable to carry out additional studies adequate sample size, with specific pathology and with personnel already specialized in the execution of geriatric tests, to confirm our findings with the intention of advancing in the knowledge of the potential impact that the geriatric evaluation can have on the treatment of elderly patients with cancer.
Abbreviations:
"CRC III": stage III colon cancer; "CRC IV": stage IV colon cancer; "NSCLC IV": stage IV non-small cell lung cancer; "IGE": comprehensive geriatric evaluation; "HR": registration historical; “KS”: Karfnosky score; “PS”: Performance status.
Conflict of Interest
All authors of the present study do not present any conflict of interest / disclosure of the content of the research presented
Authors' Information
In the manuscript, the procedures followed are in accordance with the ethical standards of the institutional and national guidelines
References
1. Marije E, Marthe M, Noortje T, et al. The effect of a geriatric evaluation on treatment decisions and outcome for older cancer patients a systematic review. J Geriatric Oncol. 2018; 9 : 430–440
2. Nancy L, Landrum M, Klabunde C, et al. Adjuvant Chemotherapy for Stage III Colon Cancer: Do Physicians Agree About the Importance of Patient Age and Comorbidity?. J Clin Oncol. 2008; 26: 2532-2537.
3. Jessup JM, Stewart A, Greene FI, et al. Adjuvant Chemotherapy for Stage III Colon Cancer Implications of Race/Ethnicity, Age, and Differentiation. JAMA. 2005; 294: 2703-2711.
4. Hubbard J, David M, Yothers G, et al. Benefits and Adverse Events in Younger Versus Older Patients Receiving Adjuvant Chemotherapy for Colon Cancer: Findings From the Adjuvant Colon Cancer Endpoints Data Set. J Clin Oncol. 2012; 30: 2334-2339.
5. De Angelis R, Sant M, Coleman MP, et al. EUROCARE-5 Working Group. Cancer survival in Europe 1999-2007 by country and age: results of EUROCARE—5 a population-based study. Lancet Oncol. 2014;15 (1):23-34.
6. Gómez-España MA, Gallego J, González-Flores E, et al. SEOM clinical guidelines for diagnosis and treatment of metastatic colorectal cancer. Clin Transl Oncol. 2018; 21: 46–54.
7. Extermann M, Hurria A, et al. Comprehensive Geriatric Assessment for Older Patients With Cancer. J Clin Oncol. 2007; 25 (14): 1824-31
8. E Van Cutsem, A Cervantes, B Nordlinger, et al. Metastasic Colorectal cáncer, ESMO clinical practice guidelines. Ann Oncol. 2014; 25 (suppl 3): iii1-iii9
9. National Comprehensive Cancer Network. Older Adult Oncology (Version 1.-2020). https://www.nccn.org/professionals/physician_gls/pdf/senior.pdf. Accessed February 7, 2020.
10. Wu, D Planchard, S Lu, H. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with metastatic non-small cell lung cancer: a CSCO-ESMO initiative endorsed by JSMO, KSMO, MOS, SSO and TOS. Ann Oncol. 2019; 30: 171–210.
11. Extermann M, Overcash J, Lyman GH, et al. Comorbidity and functional status are independent in older cancer patients. J Clin Oncol. 1998; 16:1582-87.
12. Quoix E, Zalcman G, Oster JP. Carboplatin and weekly paclitaxel doublet chemotherapy compared with monotherapy in elderly patients with advanced non-small-cell lung cancer: IFCT-0501 randomized, phase 3 trial. Lancet. 2011; 378: 1079–88
13. Maemondo M, Minegishi Y, Inoue A et al. First-line gefitinib in patients aged 75 or older with advanced non-small cell lung cancer harboring epidermal growth factor receptor mutations: NEJ 003 study. J Thorac Oncol. 2012; 7: 1417–22.
14. Rodríguez J, Tabares Z, Jiménez V, et al. Evaluación geriátrica integral, importancia, ventajas y beneficios en el manejo del adulto mayor. Panorama Cuba y Salud. 2014;9 (1):35-41.
15. Kameyama F. Evaluacion Geriatrica Integral: Revision- Punto de vista. El Residente. 2010; 2: 55-65.
16. Sanjoaquin A, Fernandez E, Mesa M, et al. Valoración geriátrica integral. Tratado de geriatría. 2009; 4: 59-68.
17. Fleming K, Evans J, Weber D, et al. Practical functional assessment of elderly persons: A primary-care approach. Mayo Clin Proc 1995; 70: 710-895
18. Midori S, Yatabe, Taguchi F, et al. Mini Nutritional Assessment as a Useful Method of Predicting the Development of Pressure Ulcers in Elderly Inpatients. J Am Geriatr Soc. 2013; 61:1698–1704.
19. Bauer, J, Vogl, T, Wicklein S, et al. Comparison Of The Mini Nutritional Assessment, Subjective Global Assessment, And Nutritional Risk Screening For Nutritional Screening And Assessment In Geriatric Hospital Patients. Z.Gerontol. Geriatr. 2002; 38: 322-327.
20. Bleda M, Bolibar I, Pares R, et al. Reliability of the mini nutritional assessment (MNA) in institutionalized elderly people. J Nutr Health Aging. 2006; 2:134-7.
21. Labianca R. Early colon cancer: ESMO Clinical practice Guidelines for diagnosis, treatment and follow up. Ann oncol. 2013; 64: 64-72.
22. National Comprehensive Cancer Network Colon cancer NCCN evidence Blocks. https://www.nccn.org/professionals/physician_gls/pdf/colon_blocks.pdf. (Version 4, 2020) Accessed june 15, 2020.
23. Folprecht G, Seymour M, Saltz L, et al. Irinotecan/fluorouracil combination in first-line therapy of older and younger patients with metastatic colorectal cancer: combined analysis of 2,691 patients in randomized controlled trials. J Clin Oncol. 2008; 26: 1443-1451
24. Sederholm C, Hillerdal G, Lamberg K, et al. Phase III trial of gemcitabine plus carboplatin versus single-agent gemcitabine in the treatment of locally advanced ormetastatic non-small-cell lung cancer: the Swedish Lung Cancer Study Group. J Clin Oncol. 2005; 23: 8380-88
25. Comella P, Frasci G, Carnicelli P, et al. Gemcitabine with either paclitaxel or vinorelbine vs paclitaxel or gemcitabine alone for elderly or unfit ad- vanced nonsmall- cell lung cancer patients. Br J Cancer. 2004; 91:489 – 497.
26. Soubeyran P, Fonck M. Predictors of Early Death Risk in Older Patients Treated With First-Line Chemotherapy for Cancer. J Clin Oncol. 2012; 30:1829-34.
27. Hurria A, Togawa K, Mohile S, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011; 29: 3457– 65.
28. Kohne CH. Chemotherapy in Elderly Patients with Colorectal Cancer. The Oncologist. 2008;13:390–402.
29. Grande R, Natoli C. Treatment of Metastatic Colorectal Cancer Patients 75 Years Old in Clinical Practice: A Multicenter Analysis. PLoS ONE. 2016; 11(7): 1-11
30. Repetto L, Fratino L, Audisio RA, et al. Comprehensive geriatric assessment adds information to Eastern Cooperative Oncology Group performance status in elderly cancer patients: an Italian Group for Geriatric Oncology Study. J Clin Oncol. 2002; 20: 494–502.
31. Sundararajan V, Mitra N, Jacobson JS, et al. Survival associated with 5-flu- orouracil-based adjuvant chemotherapy among elderly patients with node- positive colon cancer. Ann Intern Med. 2002; 136:349 –357.
32. Tanya M, Kallogjeri, Powers B, et al. The Benefit of Adjuvant Chemotherapy in Elderly Patients with Stage III Colorectal Cancer is Independent of Age and Comorbidity J Geriatr Oncol. 2010; 1(2): 48–56.
33. Potosky AL, Harlan LC, Kaplan RS, et al. Age, sex, and racial differences in the use of standard adjuvant therapy for colorectal cancer. J Clin Oncol. 2002; 20:1192–1202.
Received:August 06, 2020.
Accepted:August 13, 2020
Published:August 20, 2020.
To cite this article : Salgado AA,Fenoll SF,Garcia EC, et al. Pilot Study of Frailty Evaluation and Treatment in Elderly Cancer Patients.British Journal of Cancer Research. 2020;3:4.
©Salgado AA, et al. 2020.
