Research article / Open Access
DOI: 10.31488/bjcr.183
Results of a Phase I Study of Liposomal Annamycin for Treatment of R/R AML Patients after Induction Therapy
Lidia Gil 1,2, Agnieszka Wierzbowska3, Tomasz Wróbel4, Ewa Lech-Maranda5, Slawomir Milczarek6, Boguslaw Machalinski6, Cynthia C Abbate7, Erikson Wasyl7, Robert Shepard7, J. Paul Waymack7, Waldemar Priebe7,8, Wolfram Dempke7,9
1. Department of Haematology and Bone Marrow Transplantation; Poznan University of Medical Sciences, Poland
2. Department of Haematology, Poznan, Poland
3. Medical University of Lódz, Department of Haematology, Lódz, Poland
4. University Clinic of Wroclaw, Department of Haematology, Wroclaw, Poland
5. Institut of Haematology and Transfusion Medicine, Warsaw, Poland
6. University of Szczecin, Department of Haematology, Szczecin, Poland
7. Moleculin Biotech Inc, Houston, Texas, USA
8. The University of Texas, MD Anderson Cancer Center, Houston, Texas, USA
9. University of Munich, Medical School, Campus Grosshadern, Munich, Germany
*Corresponding author:Professor Wolfram Dempke, MD, PhD, MBA, Moleculin Biotech Inc, 5300 Memorial Drive, Suite 950, Houston, TX 77077, USA
Abstract
Therapies for r/r AML are very limited, with poor outcomes. Anthracycline-based chemotherapy administration is limited and can lead to cardiotoxicity. L-Annamycin is a novel liposomal anthracycline derivative without any cardiotoxic effects. It can overcome mdr-1-associated resistance in leukaemic blasts and is a very potent TOPO-IIα poison. We report a phase I as a multi-centre, open-label, dose-escalation trial with L-annamycin as single agent for the treatment of r/r AML patients after induction chemotherapy to determine the MTD and the RP2D. Enrollment occurred in cohorts of three patients in a conventional 3 + 3 escalating dose design, and a total of 20 patients were enrolled. Treatment with L-annamycin administered on three consecutive days was well tolerated with no cardiotoxicity recorded. The study met its primary endpoint with a RP2D of 240 mg/m² given on three consecutive days. ORR in this patient population was found to be 20% in the intent-to-treat population and 23.5% in the efficacy-evaluable population. In the highest dose cohort (240 mg/m²) ORR was found to be 80%. Since animal data showed that L annamycin in combination with cytarabine demonstrated a significant improvement in OS compared to single agent therapy, this trial was terminated after phase I part and a subsequent phase I/II trial (MB-106) is conducted in Europe (NCT05319587).
Keywords: r/r AML, liposomal annamycin, phase I, cardiotoxicity
Introduction
Even in the area of advanced knowledge of the underlying molecular biology of leukaemias, treatment approaches remain a therapeutic challenge. In particular, acute myeloid leukaemia (AML) is still associated with a high mortality rate despite the fact that advancements in molecular risk stratification and new treatment paradigms have led to new improvements in the outcomes for specific subgroups [1].
During the last four to five decades the underlying molecular alterations of the disease or the AML subtype did not significantly impact front-line induction chemotherapy. Almost all leukaemia centres have treated (and still treat) AML patients being eligible for intensive chemotherapy with an anthracycline (e.g., daunorubicin or idarubicin for 3 consecutive days) plus cytarabin (continuous infusion over 7 days) – generally known as the “7 + 3” regimen. A significant breakthrough in terms of mOS (median overall survival) benefit was recently demonstrated by a new “7 + 3” formulation (CPX-351, Vyxeos liposomal®) in patients with secondary AML [2].
Results from clinical trials evaluating immunotherapy agents (checkpoint inhibitors) in patients with acute leukaemias have been disappointing since the immune system of de novo and r/r AML patients appears to be significantly impeded prior to treatment (i.e. due to massive bone marrow infiltration – “immune desert”), checkpoint expression is very low at baseline, and clonal heterogeneity of AMLs appear to be the main causes for treatment failure of therapies relying on patient’s immune function [3].
During the last couple of years, the treatment armamentarium for AML patients has significantly changed with the development and approval of several targeted therapies (e.g., inhibitors of Flt-3, IDH1,2, bcl-2, DOT1L, Hedgehog, bromodomain, etc.), and novel targets (e.g., DNA methylation, CD33, CD47, CD70, CD123 etc.) in the first- and second-line setting [4].
Anthracyclines have contributed significantly to remarkable improvements in overall survival and are regarded as the most effective cytostatic and cytotoxic drugs for haematological malignancies. Although chemotherapy remains the backbone for all acute leukaemias, its cumulative administration is limited due to the cardiotoxic effects of anthracyclines in the regimens used. Anthracyclines are a significant cause of acute and chronic cardiotoxicity in cancer patients, and long-term cardiotoxicity can lead to death in about one third of patients. Several molecular pathways have been implicated in the development of anthracycline-induced cardiotoxicity, although the underlying mechanisms of some molecular pathways are not fully elucidated. It is now generally believed that anthracycline-induced reactive oxygen species (resulting from intracellular metabolism of anthracyclines) and drug-induced inhibition of topoisomerase IIβ are the key mechanisms responsible for the cardiotoxicity [5].
The innovative idea of combining both, liposomal formulations and novel chemical structure modifications, that could lead to reduced cardiotoxicity and increased antitumour activity against mdr-1 cancers has led to the development of L-annamycin (reviewed by [6]). An initial key modification leading to the design of L-annamycin was the replacement of a basic amine at the C-3’ position with a hydroxy group, which was shown to significantly reduce cardiotoxicity when compared with doxorubicin [7]. Removal of the basic amine from doxorubicin not only decreased cardiotoxicity, but also led to increased activity against mdr-1 tumours. In addition to the C-3’ hydroxylation, L-annamycin incorporates several important structural modifications, including demethoxylation at C-4, epimerization at C-4’, and for the first time in this class of agents, an iodine atom was introduced at C-2’ position. L-annamycin has been shown to be a consistently more potent inducer of apoptosis than doxorubicin and more efficacious in vivo against tumours expressing mdr-1 tumours [8]. Separate studies documented L-annamycin as a potent TOPO-IIα poison [7].
The promising results of L-annamycin in preclinical models and an earlier trial (MB-104) prompted us to conduct a multi-centre, open-label dose-escalation study with L-annamycin as a single therapy for the treatment of relapsed or refractory (r/r) AML patients following induction chemotherapy to determine the maximal tolerated doses (MTD) and the recommended doses for further phase II testing (RP2D).
Methods
Trial design and patients
This was a multicentre, open-label, dose-escalation study to determine the MTD and RP2D of L annamycin as a single agent for the treatment of patients with r/r AML after induction therapy.
Patients were male or female, aged ≥18 years, with a pathologically confirmed diagnosis of r/r AML following induction therapy. They were required to have adequate laboratory results and an Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2. Female patients of childbearing potential had to have a negative pregnancy test at screening and all patients had to agree to practice effective contraception throughout treatment and for defined periods after their last dose.
Eligible patients could not participate in the study if (i) they had received chemotherapy, radiation, major surgery (unless recovered from the toxic side effects of any previous therapy) or other investigational therapies within specific timeframes prior to first dose, if (ii) they were diagnosed with acute promyelocytic leukemia, if (iii) they were receiving concomitant therapy that would have been active against AML, if (iv) they had central nervous system involvement, if (v) their cardiac function did not meet specified conditions, if (vi) they had clinically relevant serious comorbid medical conditions, if (vii) they had an allergy to anthracyclines, if (viii) they had ongoing grade 1 mucositis, or if (ix) they had any other condition which, in the opinion of the investigator, made them unsuitable for this study. A full list of the inclusion and exclusion criteria for the study is presented on www.clincialtrials.gov (NCT03388749).
This study was conducted in accordance with the United States (US) Food and Drug Administration (FDA) Code of Federal Regulations, the principles of Good Clinical Practice (GCP) (current International Council for Harmonization [ICH] guidelines), the European Clinical Trial Directive 2001/20/EC, the European Union GCP Directive 2005/28/EC, and the Declaration of Helsinki (1964) including all amendments up to and including the Fortaleza, Brazil revision (2013).
Each patient was provided with oral and written information describing the nature and duration of the study in a language they could understand. They had to consent to participate in writing before undergoing screening. The date of the consent had to be entered by each patient. The original signed consent form was retained with the study centre’s records. Moreover, each patient was given a copy of his/her signed consent form.
Endpoints of the study
The primary objective of this trial was to evaluate the safety and to identify the RP2D of L-annamycin for the treatment of r/r AML patients after induction therapy.
The secondary objectives included (i) the pharmacokinetics of L-annamycin and its metabolite annamycinol, and (ii) the preliminary assessment of the anti-leukaemic activity of L-annamycin as second line (or subsequent) therapy for r/r AML patients on the basis of established response criteria, including complete response (CR), CR with incomplete recovery of platelets and/or neutrophils (CRi), partial response (PR), event-free survival (EFS), overall survival (OS), and time to and duration of response.
Treatment
This was a multicentre, open-label, dose-escalation study to determine the MTD and RP2D of L annamycin as a single agent for the treatment of patients with r/r AML after induction therapy.
Enrollment occurred in cohorts of three patients in a conventional 3 + 3 escalating dose design, starting at a dose level of 120 mg/m2/day administered for three consecutive days. Dose escalation was to take place on the basis of safety assessments in sequential cohorts of three patients each. In the absence of dose-limiting toxicities (DLTs), dose escalation by 30 mg/m2/day increments was to continue in subsequent cohorts until an MTD was reached. Thus, subsequent cohorts were to receive 150, 180, 210, and 240 mg/m2/day of L annamycin for three days in the absence of safety concerns. However, if one of the three initial patients experienced a DLT, the cohort of patients at that dose level was to be expanded to six patients. If at least two of the six patients experienced a DLT, this was considered to be a toxic dose and the next three patients were treated at a lower dose. The dose was to be de-escalated by 15 mg/m2/day. If one of the three initial patients experienced a DLT at the lowered dose, the cohort of patients was expanded to six subjects. If at least two of the six patients experienced a DLT, this was then considered to be a toxic dose.
After determination of the MTD (defined as the highest dose of L-annamycin at which fewer than two of a cohort of up to six patients experienced a DLT) and the RP2D (defined as the optimal dose to be explored in the expansion phase portion of the study as determined by the sponsor on the basis of review of available clinical and laboratory safety and efficacy data), up to 21 additional patients were to be enrolled at either the MTD or RP2D to better define toxicity and evaluate efficacy at these doses.
All patients received one initial cycle of L annamycin lasting 21 days comprised of three consecutive days of daily intravenous infusions followed by 18 days off study drug. An end of study (EOS) visit was conducted one week after the end of the initial cycle, and safety and survival follow up visits were to take place every three months thereafter.
A patient could receive one additional cycle of L annamycin after the end of the initial cycle if the patient experienced a near-CR after completion of that cycle.
Efficacy and safety assessments
Safety
Patients were hospitalized for the full three days of L-annamycin administration and for 11 days thereafter. If a patient experienced a DLT or any severe or life-threatening event occurred at any time over the first three days, dosing was interrupted until resolution of the event, or the patient was discontinued from the study on the basis of clinical assessment. These specific events included (but were not limited to) development of an anaphylactic reaction during or after the infusion, clinical or radiological evidence of left ventricular dysfunction with dyspnea and pulmonary rales, or signs of pulmonary hypertension on chest x-ray, or acute cardiotoxicity. Patients were then evaluated weekly thereafter during the initial cycle of treatment (the initial cycle consisted of 21 days [three weeks total], with the first three consecutive days of daily L-annamycin treatment followed by 18 days off L-annamycin), and if the patient was eligible, weekly during the subsequent cycle. An EOS visit was conducted one week after the end of the initial cycle or after the last study drug administration if the treatment period was prematurely terminated.
Patients could only continue treatment after the end of the initial cycle if this was judged to be in the best interest of the patients.
ECGs and documentation of concomitant medications affecting cytochrome P-450 family of enzymes were obtained to ensure that these were a consideration in the evaluation of the safety and pharmacokinetics of this drug.
All ECHO recordings were submitted to a central laboratory (Duke University School of Medicine; Cardiac Diagnostic Unit ECHO Core Lab, USA) for global longitudinal strain (GLS) analyses. Furthermore, all cardiac safety data collected on the MB 105 study were sent to an expert in assessing chemotherapy related cardiotoxicity at the Cleveland Clinic. This included the left ventricular ejection fraction (LVEF) and troponin was measured as one of the most sensitive indicators of cardiac toxicity, in addition to the ECHO GLS report(s) provided by Duke’s ECHO Core Lab.
Efficacy
The safety and efficacy measurements used in this study are widely used and considered standard, as indicated by the use of NCI CTCAE v5 and the International Working Group criteria. All patients evaluable for efficacy were assessed for response to treatment by using the recommendations of the International Working Group for standardization of response criteria, treatment outcomes, and reporting for therapeutic trials [9]. The primary efficacy variable was leukaemia response rate, evaluated by the investigator at the end of each cycle and at EOS follow-up, based on bone marrow aspirate and peripheral blood collected at the end of cycle 1 and after subsequent cycles, if administered. A bone marrow aspirate (biopsy if there were no spicules present) was repeated in one week if there was a question of residual leukaemia in assessing efficacy based on an initial bone marrow specimen.
An evaluation of response to therapy was one of the objectives of this study. Response criteria are described as follows. CR: Achievement of normal bone marrow morphology on light microscopy with fewer than 5% blasts (recovery of peripheral blood counts with an absolute neutrophil count >1.0 × 109/L and platelet counts >100 × 109/L); CRi: CR with incomplete recovery of platelets and/or neutrophils; PR: a ≥50% decrease in marrow blasts; patient deemed eligible for haematopoietic stem cell transplantation.
Pharmacokinetics
The pharmacokinetics of L-annamycin and its metabolite, annamycinol, were determined. Blood samples for pharmacokinetic analysis were to be collected at pre-dose and at 0.25, 0.5, 1, 2, 4, 8, and 24 hours after the start of L-annamycin infusion on day 1 and day 3 during the initial cycle only for three patients at each dose and six patients at the RP2D who completed the three days of dosing.
Results
Baseline characteristics and demographic of patients
A total of 20 patients were enrolled in the study at five investigational sites in Poland. All 20 subjects were treated with L-annamycin from February 2019 to February 2022 and all 20 subjects (100%) treated in the study are off study. Ten patients (50.0%) discontinued treatment due to objective disease progression, six patients (30.0%) completed treatment per protocol, two patients (10.0%) came off-study due to ‘other’ reasons (patients started new AML treatments), one patient (5.0%) discontinued treatment due to unacceptable toxicity, and one patient (5.0%) withdrew consent. Basic patient characteristics and demographic are shown in table 1 and table 2. The relevant prior AML treatments of the patients enrolled are listed in table 3.
Table 1.Baseline demographic data for patients enrolled
| Phase I L annamycin Dose Level (mg/m2/day) | ||||||
|---|---|---|---|---|---|---|
| Demographics | 120 | 150 | 180 | 210 | 240 | Overall |
| Number of subjects | 3 | 3 | 3 | 3 | 8 | 20 |
| Age (years) | ||||||
| N | 3 | 3 | 3 | 3 | 8 | 20 |
| Mean | 57.7 | 57.7 | 59.3 | 68.7 | 64.3 | 62.2 |
| Standard Deviation | 8.96 | 29.19 | 6.43 | 6.03 | 10.66 | 12.82 |
| Median | 53.0 | 73.0 | 62.0 | 68.0 | 66.5 | 64.5 |
| Minimum | 52 | 24 | 52 | 63 | 40 | 24 |
| Maximum | 68 | 76 | 64 | 75 | 73 | 76 |
| Age group (years) | ||||||
| 18 to 64 | 2 (66.7%) | 1 (3.3%) | 3 100.0%) | 1 (33.3%) | 3 (37.5%) | 10 (50.0%) |
| 65 + | 1 (33.3%) | 2 (66.7%) | 0 | 2 (66.7%) | 5 (62.5%) | 10 (50.0%) |
| Sex | ||||||
| Female | 1 (33.3%) | 2 (66.7%) | 0 | 2 (66.7%) | 5 (62.5%) | 11 (50.0%) |
| Male | 2 (66.7%) | 0 | 1 (33.3%) | 3 (100%) | 3 (37.5%) | 9 (45.0%) |
| Race | ||||||
| White | 3 (100%) | 3 (100%) | 3 (100%) | 3 (100%) | 8 (100%) | 20 (100%) |
| ECOG Performance Status | ||||||
| 0 | 1 (33.3%) | 0 | 0 | 1 (33.3%) | 4 (50.0%) | 6 (30.0%) |
| 1 | 2 (66.7%) | 3 (100%) | 3 (100%) | 2 (66.7%) | 3 (7.5%) | 13 (65.0%) |
| 2 | 0 | 0 | 0 | 0 | 1 (12.5%) | 1 (5.0%) |
Table 2.Baseline disease characteristics at diagnosis before enrollment
| Phase I L annamycin Dose Level (mg/m2/day) | ||||||
|---|---|---|---|---|---|---|
| Baseline disease characteristics | 120 | 150 | 180 | 210 | 240 | Overall |
| Number of subjects | 3 | 3 | 3 | 3 | 8 | 20 |
| AML types included in study [1] | ||||||
| AML relapsed after standard induction therapy | 1 (33.3%) | 1 (33.3%) | 2 (66.7%) | 1 (33.3%) | 6 (75.0%) | 11 (55.0%) |
| AML refractory after standard induction therapy | 2 (66.7%) | 2 (66.7%) | 1 (33.3%) | 2 (66.7%) | 2 (25.0%) | 9 (45.0%) |
| Duration of disease (months) [2] | ||||||
| N | 3 | 3 | 3 | 3 | 8 | 20 |
| Mean | 18.6 | 6.6 | 15.6 | 11.2 | 13.0 | 13.0 |
| Standard Deviation | 7.8 | 5.9 | 14.3 | 7.1 | 11.8 | 10.5 |
| Minimum | 2 | 3 | 7 | 4 | 6 | 2 |
| Maximum | 46 | 11 | 25 | 23 | 23 | 46 |
| [1] Number of patients used as denominator to calculate percentages.
[2] Duration of disease was calculated from the date of initial diagnosis to the first dose date. |
||||||
Table 3.Relevant prior AML therapies for patients in the intent-to-treat population
| Phase I L annamycin Dose Level (mg/m2/day) | ||||||
|---|---|---|---|---|---|---|
| Prior AML therapy [2] | 120 | 150 | 180 | 210 | 240 | Overall |
| Number of patients | 3 | 3 | 3 | 3 | 8 | 20 |
| Prior chemotherapy [1] | 3 (100%) | 3 (100%) | 3 (100%) | 3 (100%) | 8 (100%) | 20 (100%) |
| 1 Regimen | 1 (33.3%) | 0 | 0 | 1 (33.3%) | 0 | 2 (10.0%) |
| 2 Regimens | 0 | 2 (66.7%) | 0 | 0 | 1 (12.5%) | 3 (15.0%) |
| ≥ 3 Regimens | 2 (66.7%) | 1 (33.3%) | 3 (100.0%) | 2 (66.7%) | 7 (87.5%) | 15 (75.0%) |
| N | 3 | 3 | 3 | 3 | 8 | 20 |
| Mean | 4.3 | 2.3 | 3.3 | 2.7 | 7.5 | 4.9 |
| Standard Deviation | 4.16 | 0.58 | 0.58 | 1.53 | 4.66 | 3.91 |
| Median | 3.0 | 2.0 | 3.0 | 3.0 | 6.5 | 3.5 |
| Minimum | 1 | 2 | 3 | 1 | 2 | 1 |
| Maximum | 9 | 3 | 4 | 4 | 18 | 18 |
| Prior immunotherapy [1] | 0 | 0 | 0 | 0 | 1 (12.5%) | 1 (5.0%) |
| 1 Regimen | 0 | 0 | 0 | 0 | 1 (12.5%) | 1 (5.0%) |
| N | 0 | 0 | 0 | 0 | 1 | 1 |
| Mean | 1.0 | 1.0 | ||||
| Median | 1.0 | 1.0 | ||||
| Minimum | 1 | 1 | ||||
| Maximum | 1 | 1 | ||||
| Other prior therapies [1] | 0 | 0 | 2 (66.7%) | 0 | 0 | 2 (10.0%) |
| 1 Regimen | 0 | 0 | 2 (66.7%) | 0 | 0 | 2 (10.0%) |
| N | 0 | 0 | 2 | 0 | 0 | 2 |
| Mean | 1.0 | 1.0 | ||||
| Standard Deviation | 0.00 | 0.00 | ||||
| Median | 1.0 | 1.0 | ||||
| Minimum | 1 | 1 | ||||
| Maximum | 1 | 1 | ||||
| Prior anthracycline therapy [1] | 3 (100.0%) | 2 (66.7%) | 3 (100.0%) | 3 (100.0%) | 5 (62.5%) | 16 (80.0%) |
| Prior transplant [1] | 0 | 0 | 1 (33.3%) | 0 | 0 | 1 (5.0%) |
| [1] Number of patients used as denominator to calculate percentages.
[2] Patients may be counted in more than one prior therapy category. |
||||||
Safety
All 20 patients enrolled (100.0%) received at least one dose of treatment, comprising the safety evaluable population and all 20 patients (100.0%) and at least one treatment emergent adverse event (TEAE) of any severity. A total of 18/20 patients (90%) experienced at least one severe TEAE (grade 3) and 15/20 patients (75%) were found to have at least one drug related severe TEAE.
A total of 17 patients (85.0%) experienced at least one serious adverse event (SAE) in the study and 11/20 patients (55%) had a least one SAE that was possibly, probably or definitely related to study drug.
Two patients (10.0%) experienced TEAEs leading to death, including one TEAE of corona virus infection (grade 5) and one TEAE of multiple organ dysfunction (grade 5), both assessed as unrelated to study drug (Table 4), but considered as DLTs.
Table 4.Overall summary of treatment-related adverse events
| Phase I L annamycin Dose Level (mg/m2/day) | ||||||
|---|---|---|---|---|---|---|
| Treatment-emergent adverse events [1] | 120 | 150 | 180 | 210 | 240 | Overall |
| Number of patients | 3 | 3 | 3 | 3 | 8 | 20 |
| Patients | ||||||
| with any TEAEs[1][2] | 3 (100.0%) | 3 (100.0%) | 3 (100.0%) | 3 (100.0%) | 8 (100.0%) | 20 (100.0%) |
| with drug-related [3] TEAEs [1][2] | 3 (100.0%) | 3 (100.0%) | 3 (100.0%) | 3 (100.0%) | 8 (100.0%) | 20 (100.0%) |
| with grade 3, 4 or 5 [4] TEAEs [1][2] | 3 (100.0%) | 3 (100.0%) | 3 (100.0%) | 3 (100.0%) | 6 (75.0%) | 18 (90.0%) |
| with grade 3, 4 or 5 [4] drug-related [3] TEAEs [1][2] | 3 (100.0%) | 3 (100.0%) | 3 (100.0%) | 2 (66.7%) | 4 (50.0%) | 15 (75.0%) |
| with any serious TEAEs [1][2] | 2 (66.7%) | 3 (100.0%) | 2 (66.7%) | 3 (100.0%) | 7 (87.5%) | 17 (85.0%) |
| with any serious, drug-related [3] TEAEs [1][2] | 2 (66.7%) | 3 (100.0%) | 1 (33.3%) | 1 (33.3%) | 4 (50.0%) | 11 (55.0%) |
| with drug withdrawn due to TEAEs [1][2] | 0 | 0 | 0 | 0 | 3 (37.5%) | 3 (15.0%) |
| who died due to any TEAE [1][2] | 0 | 0 | 0 | 0 | 2 (25.0%) | 2 (10.0%) |
| [1] TEAEs were defined as AEs that occurred on or after the first dose of study drug up to 30 days post last dose. SAEs were captured from first dose of study drug through 30 days after the last dose of study drug or any SAE occurring later than 30 days following last dose if investigator believed it was related to study drug.
[2] Number of patients used as denominator to calculate percentages. [3] Drug related includes L-annamycin relationship as Definite, Probable, Possible. [4] Grade: 1 = Mild, 2 = Moderate, 3 = Severe, 4 = Life threatening, 5 = Fatal. TEAE: treatment-emergent adverse events |
||||||
No evidence of cardiac toxicity was identified. Both cardiac enzyme concentrations (troponins) and LVEF remained stable throughout the study in all patients. Independent review of the cardiac safety data confirmed that there was no evidence of cardiotoxicity in any patient treated in this study, including up to 16 patients whose cumulative anthracycline dose (L annamycin included) exceeded the lifetime cumulative doxorubicin (or equivalent) dose of > 450 mg/m2. To that end, the cardiac toxicity, seen with other anthracyclines, has not yet been identified with L-annamycin.
A summary of drug-related treatment-emergent adverse events (occurring in > 10% of patients) is shown in table 5.
Table 5.Drug-related treatment-emergent adverse events (occurring in > 10% of patients)
| Phase I L annamycin Dose Level (mg/m2/day) | ||||||
|---|---|---|---|---|---|---|
| MedDRA System Organ Class MedDRA Preferred Term [1] | 120 | 150 | 180 | 210 | 240 | Overall |
| Number of patients | 3 | 3 | 3 | 3 | 8 | 20 |
| Patients with any drug related [4] TEAEs [2][3] | 3 (100.0%) | 3 (100.0%) | 3 (100.0%) | 3 (100.0%) | 8 (100.0%) | 20 (100.0%) |
| Blood and lymphatic system disorders | 3 (100.0%) | 3 (100.0%) | 3 (100.0%) | 2 (66.7%) | 4 (50.0%) | 15 (75.0%) |
| Neutropenia | 3 (100.0%) | 3 (100.0%) | 3 (100.0%) | 1 (33.3%) | 3 (37.5%) | 13 (65.0%) |
| Thrombocytopenia | 3 (100.0%) | 3 (100.0%) | 2 (66.7%) | 1 (33.3%) | 1 (12.5%) | 10 (50.0%) |
| Anaemia | 1 (33.3%) | 3 (100.0%) | 3 (100.0%) | 1 (33.3%) | 1 (12.5%) | 9 (45.0%) |
| Febrile neutropenia | 1 (33.3%) | 1 (33.3%) | 2 (66.7%) | 1 (33.3%) | 3 (37.5%) | 8 (40.0%) |
| Pancytopenia | 1 ( 33.3%) | 3 (100.0%) | 0 | 0 | 0 | 4 (20.0%) |
| Gastrointestinal disorders | 2 (66.7%) | 2 (66.7%) | 0 | 1 (33.3%) | 1 (12.5%) | 6 (30.0%) |
| Nausea | 2 (66.7%) | 0 | 0 | 1 (33.3%) | 0 | 3 (15.0%) |
| General disorders and administration site conditions | 2 (66.7%) | 1 (33.3%) | 3 (100.0%) | 0 | 0 | 6 (30.0%) |
| Pyrexia | 1 (33.3%) | 1 (33.3%) | 2 (66.7%) | 0 | 0 | 4 (20.0%) |
| Mucosal inflammation | 1 (33.3%) | 1 (33.3%) | 1 (33.3%) | 0 | 0 | 3 (15.0%) |
| Hepatobiliary disorders | 1 (33.3%) | 0 | 0 | 0 | 3 (37.5%) | 4 (20.0%) |
| Investigations | 2 (66.7%) | 0 | 1 (33.3%) | 0 | 1 (12.5%) | 4 (20.0%) |
| Immune system disorders | 1 ( 33.3%) | 1 ( 33.3%) | 1 ( 33.3%) | 0 | 0 | 3 (15.0%) |
| Anaphylactic reaction | 1 ( 33.3%) | 1 ( 33.3%) | 1 ( 33.3%) | 0 | 0 | 3 (15.0%) |
| Infections and infestations | 1 (33.3%) | 0 | 0 | 1 (33.3%) | 1 (12.5%) | 3 (15.0%) |
|
[1] MedDRA version 20.0 or higher
[2] Number of patients used as denominator to calculate percentages. [3] TEAEs were defined as AEs that occurred after the first dose of study drug up to 30 days post last dose. Patients with multiple TEAEs were only counted once within a summary category: system organ class, preferred term. Patients with events in more than one category were counted once within each category. [4] Drug related includes L-Annamycin relationship as Definite, Probable, Possible. |
||||||
Table 6.Best overall response rate (ORR) for the intent-to-treat population (N = 20) (A); best ORR for the efficacy-evaluable population (N = 17) (B)
| Phase I L annamycin Dose Level (mg/m2/day) | ||||||
|---|---|---|---|---|---|---|
| Best Overall response | 120 | 150 | 180 | 210 | 240 | Overall |
| Number of patients | 3 | 3 | 3 | 3 | 8 | 20 |
| Best overall response | ||||||
| CRi | 0 | 0 | 0 | 0 | 3 (37.5%) | 3 (15.0%) |
| PR | 0 | 0 | 0 | 0 | 1 (12.5%) | 1 (5.0%) |
| Treatment failure | 3 (100.0%) | 3 (100.0%) | 3 (100.0%) | 3 (100.0%) | 4 (50.0%) | 16 (80.0%) |
B
| Phase I L annamycin Dose Level (mg/m2/day) | ||||||
|---|---|---|---|---|---|---|
| Best Overall response | 120 | 150 | 180 | 210 | 240 | Overall |
| Number of patients | 3 | 3 | 3 | 3 | 5 | 17 |
| Best overall response | ||||||
| CRi | 0 | 0 | 0 | 0 | 3 (60.0%) | 1 (17.6%) |
| PR | 0 | 0 | 0 | 0 | 1 (20.0%) | 3 (5.9%) |
| Treatment failure | 3 (100.0%) | 3 (100.0%) | 3 (100.0%) | 3 (100.0%) | 1 (20.0%) | 13 (76.5%) |
Efficacy
Efficacy results are provided for both the intent-to-treat analysis set and the efficacy evaluable analysis set with results shown in tables 6A and 6B. Collectively, the ORR in this heavily pre-treated population was found to be 20% and 23.5%, respectively. Among the eight patients treated in the final dosing cohort (240 mg/m2) five were evaluable for efficacy and there were one PRs and three CRi among these five patients.
Event-free survival (EFS), mOS, time to response, duration of response, and cytogenetic CR data are limited as this study was prematurely terminated.
Pharmacokinetics (PK)
The exposure of annamycin and annamycinol for day 1 and day 3 were similar within each dose level and increased with increasing dose. The median time to maximum plasma concentration was similar across dose levels. The t1/2, and AUCinf could not be determined for the majority of patients due to either the adjR2 < 0.8 or the AUC% extrapolated greater than 20%. The concentration profiles for both compounds are depicted in figure 1.
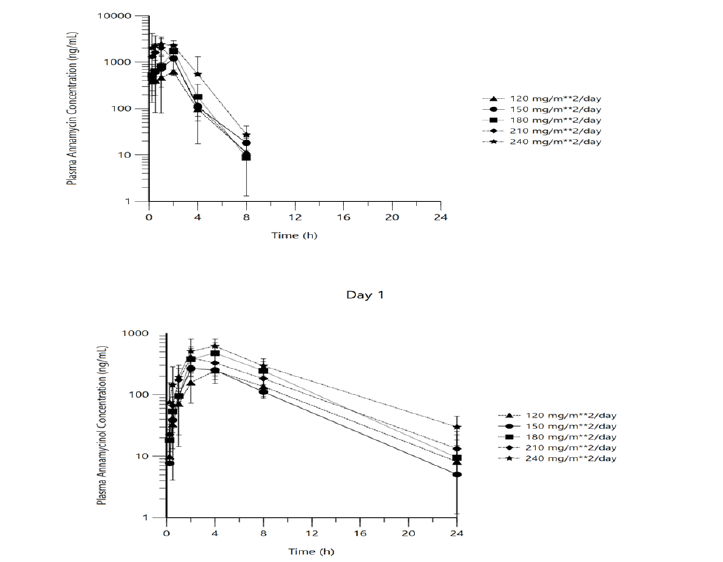
Figure 1:Mean (± SD) L-annamycin (top panel) and annamycinol (bottom panel) concentration profiles (day 1, evaluable pharmacokinetic population) (semi-log scale)
Discussion
Treatment of r/r AML patients is a major challenge and represents a high unmet medical need [10]. Although induction chemotherapy (“7 + 3”) can achieve remissions in many older AML patients, relapse is common, and the overall prognosis is very poor. Moreover, older patients and those with a significant cardiac impairment (due to prior anthracycline administration) and other co-morbidities are often not eligible for induction chemotherapy or intensive salvage therapies [11,12].
In an attempt to further investigate the clinical activity of the novel liposomal anthracycline derivative L-annamycin that was found to have no cardiotoxicity [6], a phase I trial with r/r AML patients was conducted. Of all patients enrolled, 17 (85.0%) were eligible for response assessment, comprising the efficacy evaluable population.
The most frequent AEs considered to be related to L annamycin were in the system organ classes of blood and lymphatic disorders (75.0%) and gastrointestinal disorders (30.0%). SAEs were predominantly haematologic in nature which is expected in this patient population. The study revealed that L-annamycin was well tolerated at 120, 150, 180, 210, and 240 mg/m2/day for three consecutive days, with one suspected unexpected serious adverse reaction (infusion related reaction) reported in the final cohort (240 mg/m2/day). Reported SAEs were clinically manageable and aligned with clinical safety observations in prior and ongoing clinical studies.
L-Annamycin again demonstrated no evidence of cardiotoxicity based on review of cardiotoxicity biomarkers, LVEF, and ECHO GLS evaluation. These properties differentiate L-annamycin from all other anthracyclines that have shown limited or no cardiotoxicity to date and highlight it as a very promising anticancer agent and it retains the ability to poison TOPO-IIα (topoisomerase II) and can also overcome mdr-1-related resistance mechanisms in leukaemic blasts, as shown in its parent compound [7,8]. L-Annamycin is currently undergoing several early-phase clinical trials in different indications.
Of great interest, however, is the development of L-annamycin (and other compounds with a reduced cardiotoxicity profile) for the therapy of children and younger adults with tumours that are treated with a curative intent (e.g. chondrosarcomas, osteosarcomas, Ewing sarcomas, Hodgkin’s disease, malignant lymphomas, acute leukaemias, adjuvant breast cancer, etc.) in which the elimination of long-term anthracycline-induced cardiotoxicity is still a high unmet medical need [6].
Of the 17 efficacy-evaluable patients, three patients experienced a CRi and one patient had a PR. All four of these responding patients received L annamycin at 240 mg/m2/day for three consecutive days making the ORR at the RP2D 80%. One patient who experienced a CRi qualified to have a stem cell transplant following cycle 1; however, due to administrative/scheduling issues in booking the patient in for transplant, this was not able to be performed prior to developing objective disease progression.
Based on the observed ORR of 80% (4/6: (3 CRi, 1 PR in the 240 mg/m² cohort) with upcoming DLT-related toxicities in this cohort, the DSMB (Data and Safety Monitoring Board) of this trial followed the advice of the Independent Ethics Committee (IEC) not to enroll another cohort (270 mg/m²) for ethical and safety reasons. Therefore, given the safety and efficacy data obtained in this study, it was determined that the primary objective of evaluating safety had been met by establishing the RP2D of 240 mg/m2/day for three consecutive days.
Collectively, L-annamycin demonstrated very promising clinical efficacy in heavily pre-treated r/r AML patients and appeared to be well tolerated (RP2D: 240 mg/m² x 3 days). Of particular interest is the observation that L-annamycin did not show any cardiac toxicity in these patients who had received prior anthracyclines suggesting that the drug can also be administered when the cumulative threshold of other anthracyclines has been reached (i.e., 450 mg/m² for doxorubicin).
Although preliminary efficacy data were promising at this RP2D, preclinical animal data have shown that L annamycin in combination with cytarabine demonstrated a significant improvement in mOS compared to both L annamycin as a single agent (68% improvement) and to cytarabine alone (241% increase) [13]. This observation is well in line with the hypothesis that doublet chemotherapy (e.g., venetoclax plus azacitidine) is much more potent in r/r AML patients or in AML patients not eligible for intensive chemotherapy than monotherapy [14-16].
As a result, the strategic decision was made to terminate the MB 105 single agent trial after completion of the phase I part and to proceed with evaluating L annamycin in combination with cytarabine in a phase I/II clinical trial to be conducted in Europe (NCT05319587). The decision to terminate the study prior to the expansion phase was strategic in nature and not due to any safety concerns. The subsequent combination trial (MB-106) is currently recruiting patients and open for enrollment in Europe. Results are eagerly awaited.
Acknowledgement
The study was funded by Moleculin Biotech, Inc. (Houston, Texas, USA). The authors are indebted Martin-Schmidt-Hieber, MD, PhD (Medical Clinic II, Haematology and Oncology), Carl-Thiem Clinic Cottbus (Germany), for critical reading of the manuscript.
Data Availability Statement
Materials including raw data described in this paper are provided upon request and are freely available to any researcher wishing to use them on a non-commercial basis.
Competing Interest Statements
Drs. Gil, Wierzbowska, Wróbel, Lech-Maranda, Milczarek, and Machalinski declare no conflicts of interest. Drs. Abbate, Wasyl, Shepard, and Dempke are employees and shareholders of Moleculin Biotech Inc. (Houston, Texas, USA). Dr. Priebe is a consultant and shareholder of Moleculin Biotech Inc (Houston, Texas, USA).
Author Contributions
LG: Lead Principal Investigator, enrolled patients and provided feedback to the publication strategy.
AW: Principal Investigator, enrolled patients.
TW: Enrolled patients, provided the literature search and did the pre-check of the raw clinical data.
ELM: Enrolled patients, provided the literature search and helped with the raw clinical data processing.
SM: Enrolled patients and was involved in strategy discussions.
BM: Enrolled patients and was involved strategy discussions.
CA: Provided the literature search and did the pre-check of the raw clinical data
EW: Pre-check of raw clinical data
RS: Provided feedback and was responsible for the fidelity of the data.
JPW: Provided feedback and was responsible for the fidelity of the data.
WP: Provided the help with the study design, did the PK analyses.
WD: Provided feedback and wrote the manuscript.
References
1. Dempke WCM, Desole M, Chiusolo P, Sica S, Schmidt-Hieber M. Targeting the undruggable – Menin inhibitors ante portas. J Cancer Res Clin Oncol. Doi: 10.1007/s00432-023-04752-9.
2. Lancet JE, Uy GL, Cortes JE, Newell LF, Lin TL, Ritchie EK, et al. CPX-351 (cytarabine and daunorubicin) liposome for injection versus conventional cytarabine plus daunorubicin in older patients with newly diagnosed secondary acute myeloid leukemia. J Clin Oncol. 2018; 36:2684-2692.
3. Ghosh A, Barba P, Perles MA. Checkpoint inhibitors in AML: are we there yet? Br J Haematol. 2020; 188:159-167.
4. Stubbins RJ, Francis A, Kuchenbauer F, Sanford D. Management of acute myeloid leukemia. A review for general practitioners in oncology. Curr Oncol. 2022; 29:6245-6259.
5. Bhagat A, Kleinerman ES. Anthracycline-induced cardiotoxicity: causes, mechanisms, and prevention. Adv Exp Med Biol. 2020; 1257:181-192.
6. Dempke WCM, Zielinski R, Winkler C, Silberman S, Reuther S, Priebe W. Anthracycline-induced cardiotoxicity – are we about to clear this hurdle? Eur J Cancer. 2023; 185:94-104.
7. Trevino AV, Woynarowska BA, Herman TS, et al. Enhanced topoisomerase II targeting by annamycin and related 4-demethoxy anthracycline analogues. Mol Cancer Ther. 2004; 3:1403–1410.
8. Priebe W, Van NT, Burke TG, et al. Removal of the basic center from doxorubicin partially overcomes multidrug resistance and decreases cardiotoxicity. Anti-Cancer Drugs. 1993; 4:37–48.
9. Celutkiené J, Pudil R, Lópéz-Fernandéz T, et al. Role of cardiovascular imaging in cancer patients receiving cardiotoxic therapies: a position statement on behalf of the Heart Failure Association (HFA), the European Association of Cardiovascular Imaging (EACVI) and the Cardio-Oncology Council of the European Society of Cardiology (ESC). Eur J Heart Fail. 2020; 22:1504–1524.
10. Ganzel C, Sun Z, Cripe LD, Fernandez HF, Douer D, Rowe JM, et al. Very poor long-term survival in past and more recent studies for relapsed AML patients: the ECOG-ACRIN experience. Am J Hematol. 2018; 93:1074-1081.
11. Megias-Vericat JE, Martinez-Cuadron D, Sanz MA, Montesinos P. Salvage regimens using conventional chemotherapy agents for relapsed/refractory adult AML patients: a systematic literature review. Ann Hematol. 2018; 97:1115-1153.
12. DeWolf S, Tallman MS. How I treat relapsed or refractory AML. Blood. 2020; 136:1023-1032.
13. Zal T, Zielinski R, Grela K, Cardenas-Zuniga R, Skora S, Fokt I, et al. High efficacy of liposomal annamycin (L-ANN) in combination with cytarabine in syngeneic p53-null AML mouse model. Blood (2020); 136 (Suppl 1): 6-7.
14. DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020; 383: 617-629.
15. Heuser M, Smith BD, Fiedler W, Sekeres MA, Montesinos P, Leber B, et al. Clinical benefit of glasdegib plus low-dose cytarabine in patients with de novo and secondary acute myeloid leukemia. Long-term analysis of a phase II randomized trial. Ann Hematol. 2021; 100: 1181-1194.
16. Daver NG, Dail M, Garcia J, Jonas BA, Yee KWL, Kelly KR, et al. Venetoclax and idasanutlin in relapsed/refractory AML: a randomized, open-label phase 1b trial. Blood. 2023; 1265-1276.
Received: May 15, 2023;
Accepted: June 05, 2023;
Published: June 12, 2023.
To cite this article : Gil L, Wierzbowska A, Wróbel T, Maranda EL, Milczarek S, Machalinski B, Abbate CC, Wasyl E, Shepard R, Waymack JP, Priebe W, Dempke W. The Effect of Transcutaneous Electrical Nerve Stimulation on Chemotherapy Induced Neuropathic Pain and Mental Health in a Patient with Classic Hodgkin Lymphoma. British Journal of Cancer Research. 2023; 6(1): 613- 622. doi: 10.31488/bjcr.183.
©2023 Gil L, et al.
