Research article / Open Access
DOI: 10.31488/bjcr.170
Successes in the Evolution of Front-Line Therapy in Pediatric Acute Promyelocytic Leukemia
Anna Maria Testi*1, Paolo Musiu1, Alfonso Piciocchi2, Daniela Diverio1, Maria Luisa Moleti1, Robin Foà1
1. Hematology, Department of Translational and Precision Medicine, 'Sapienza' University, Rome
2. GIMEMA Data Center, GIMEMA Foundation, Rome
*Corresponding author: Anna Maria Testi, MD, Hematology, Department of Translational and Precision Medicine, Sapienza University of Rome, Via Benevento 6, 00161 Rome, Italy, Tel: +39-06-49974739; Fax: +39-06-44241984
Abstract
Since 1993, three consecutive specific disease-tailored protocols combining all-trans retinoic acid (ATRA) and anthracyclines have been carried out in Italy for pediatric APL; the first two trials included both children and adults and the last one was conducted in the framework of the International Consortium for Childhood APL (nine different groups/countries) only for the treatment of children and adolescents with APL. Results of these studies clearly demonstrated that induction combination ATRA and anthracyclines, and more precisely idarubicin, is highly effective in APL (remission rate 96-97%; induction deaths 3-4%; no refractory); ATRA extended consolidation improves results in all patients and the combination of intermittent maintenance ATRA and chemotherapy appears to be particularly useful for patients with higher leukocyte count at diagnosis. The presenting leukocyte count significantly affects patients’ outcome. Relapse risk groups based on value of leukocyte count has led to the design of risk-adapted trials in which intensity of consolidation varied according to the risk category (standard and high-risk). Molecular bone marrow assessment by quantitative reverse transcriptase polymerase chain reaction (RQ-PCR) for the PML–RARA fusion transcript, at the end of consolidation, is the major treatment objective in APL and provides more powerful predictor of relapse than presenting leucocyte count. More recently, another differentiating agent, arsenic-trioxide (ATO), has been an additional step in the successful story of APL treatment; ATO combined with ATRA without systemic chemotherapy has resulted highly effective and allows a significant spare of toxicity at least in patients defined as standard-risk. Current studies with chemotherapy-free approaches are aimed at reducing therapy-related acute and long-term toxicities while maintaining high cure rates. The early results appear promising.
Introduction
Acute promyelocytic leukemia (APL) is a rare disease especially in the pediatric age, accounting for only 5-10% of childhood acute myeloid leukemias (AML) [1-4]. A higher frequency (about 20% of AML) is reported in children of Latino/Hispanic descent; in Italy, 16-18 pediatric patients are registered each year within the Associazione Italiana Ematologia Oncologia Pediatrica (AIEOP) [3,4].
APL occurs equally in males and females among all age groups. Risk factors associated with APL development have been investigated in adults. Case reports of therapy-related APL following etoposide for other cancers have been reported and recent studies suggest that obesity increases the risk for APL [5,6].
APL is characterized by the t(15;17) translocation, which results in the PML-RARA gene fusion that drives the development of the leukemia. The progressive unraveling of the biology of APL has led over the years to a revolution in the management of the disease which has allowed a marked improvement in the outcome of APL, making it now the most curable form of AML in both children and adults [1-4].
The success in APL treatment is due on the one side to the depth of our understanding of the driver PML-RARA mutation and on the other side to the collaborative efforts to quickly introduce and maximize the benefit of innovative therapeutic strategies.
The PML-RARA protein product has been identified as the primary driver responsible for nearly all cases of APL. This has enhanced the understanding of the mechanisms by which PML-RARA leads to APL, which, in turn, has profoundly changed the therapeutic approach to the disease and its outcome. Patients with APL nowadays experience the highest cure rates within pediatric AML, with an average overall survival (OS) near 95% and event-free survival (EFS) of 90% due to the combined use of all-trans retinoic acid (ATRA) and arsenic trioxide (ATO) to induce the terminal differentiation of APL blasts [7,8].
Nonetheless, some APL patients still experience disease and treatment complications, with early deaths (ED) prior to or shortly after the initiation of therapy accounting for the majority of fatalities, particularly in patients with presenting high white blood cell (WBC) count [9-11]. These ED are typically not included in EFS and OS rates as some patients are not adequately diagnosed and treated, and others die before being enrolled in a clinical trial. As a consequence, the true survival rate of patients with APL is lower than reported. ED are often due to bleeding complications related to the coagulopathy that frequently occurs at diagnosis or to the complications of the differentiation syndrome (DS), caused by excessive numbers of maturing myeloid cells during the first two weeks of treatment with differentiation agents [11,12]. In addition to ED, the use of cytotoxic chemotherapy, such as cytarabine and anthracyclines, further exposes patients to severe treatment side effects such as cardiotoxicity and prolonged neutropenia with an increased risk of fatal infections [13].
Current studies are therefore aimed at reducing therapy-related acute and long-term toxicities while maintaining high cure rates. The early results appear promising.
While APL is considered a favorable cytogenetic AML subgroup, additional risk groups within APL have been defined that have translated into risk-adapted treatment strategies. The WBC count at diagnosis has proven to be the most effective predictor of outcome; patients presenting with a WBC count<10x109/L are considered to be at standard risk (SR), whereas those with a WBC count 10x109/L are categorized as being at high risk (HR) of relapse and ED [14].
First-line Treatment of Pediatric APL. Role of ATRA
Prior to the characterization of APL as a unique disease as we know it today (with a defined genetic mutation), APL was treated with conventional chemotherapy (cytarabine and daunorubicin) as for the other AML types. Remission rates were lower than 60% with high rates of induction deaths due to bleeding and infectious complications [15]. In 1973, Bernard et al. [16] demonstrated that APL leukemic cells were highly sensitive to anthracyclines (daunorubicin) that yielded a complete remission (CR) rate of 19 (58%) in 33 evaluable patients with APL. Since then, anthracyclines have appeared to be the most important chemotherapeutic agent in APL management, though the anthracycline used in treatment plans could vary.
Following the discovery of PML-RARA, the use of differentiation therapy with ATRA has significantly improved outcome and has become the standard of care. ATRA binds to PML-RARA, inducing a conformational change leading to the recruitment of a proteasome and subsequent degradation of the fusion protein. This allows wild-type RARA to resume its normal function, thereby driving the differentiation of the promyelocytic leukemia cell into a mature myeloid cell.
The majority of APL clinical trials allowed the inclusion of pediatric patients, but enrolled primarily adult population. When ATRA was introduced over three decades ago, it was first used as a single agent and provided CR rates of 75-85% in adults [17,18]. However, relapses remained common and thus combination therapy with both ATRA and conventional chemotherapy became standard of care. Using this combined strategy, patients of all ages began to experience relapse rates of less than 10% at 2 years [19-21].
Numerous studies have attempted to optimize chemotherapy regimens and identify the most essential agents to improve outcome. Several clinical trials have been performed with various combinations of ATRA and chemotherapy in an attempt to obtain more durable remissions and reduce ATRA-related toxicity. As demonstrated by the European APL 91 Group randomized trial, these combinations clearly improved the disease-free survival (DFS) of APL patients compared with the results achieved with chemotherapy alone [19].
The results of the Italian multicenter GIMEMA (Gruppo Italiano Malattie EMatologiche dell’Adulto) studies in adult and pediatric APL, have demonstrated that anthracyclines alone are equally effective in inducing CRs as polychemotherapy combinations. In particular, induction monochemotherapy with a single course of idarubicin was associated with a higher CR and EFS rates in newly diagnosed APL (Figure 1) [22]. For this reason, in 1993, a protocol that combined ATRA and Idarubicin (AIDA0493 protocol) was designed by the GIMEMA, first as a pilot study and, thereafter, in association with the AIEOP, as a large multicenter study for the treatment of APL in adults and children (Figure 2 and 3) [3,23,24]. Patients in hematological CR (HCR) received 3 monthly consolidation courses mainly consisting of cytarabine and anthracyclines and, at recovery from the third course, those who tested polymerase-chain-reaction (PCR)- for the PML-RARA hybrid gene were randomized to four maintenance arms: (1) oral mercaptopurine and weekly intramuscular methotrexate; (2) ATRA for 15 days, every 3 months; (3) arm 1 for 3 months, followed by arm 2 for 15 days; and (4) no therapy. Each maintenance arm had to be repeated for a total of 2 years. After April 1997, arms 1 and 4 were closed, and patients were randomized into arms 2 and 3, which included ATRA. Patients who proved PCR+ at the end of consolidation, if eligible, underwent either an allogeneic hematopoietic stem cell transplant (HSCT) or an autologous transplant.
Between January 1993 and June 2000, 124 consecutive Italian patients younger than 18 years were registered (Table 1). One-hundred and ten were eligible for treatment; a HCR was achieved in 96% of children and 4 died during induction. No child failed to respond to induction (Table 3). ATRA was administered for a median of 32 days (range 1-56) during induction; the OS and EFS were 89% (95%, c.i. 83.0%-95.3%) and 76% (95%, c.i. 65%-85%) at 121 months, respectively (Figure 4). Univariate analysis showed that the presenting WBC count significantly affected EFS: EFS was 83% for patients with a presenting WBC count<10x109/L, compared with 59% for those with a WBC count10x109/L (p=.069) (Figure 2). The DFS for children randomized to receive ATRA+chemotherapy as maintenance was significantly better compared to that of children who received ATRA alone (77% vs 42%, respectively; p=.0177) [3].
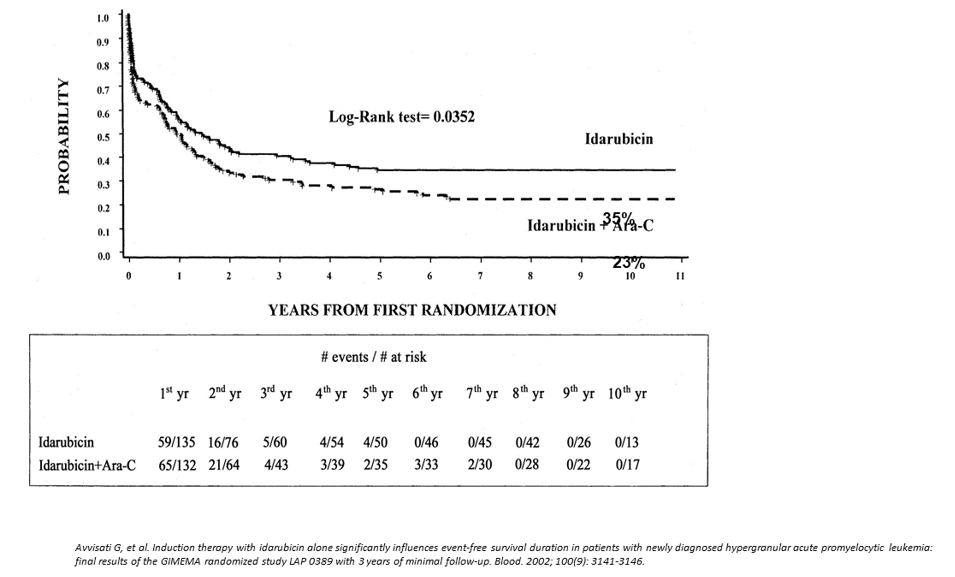
Figure 1:GiMEMA APL Italian protocol 0389. Idarubicin vs idarubicin + cytarabine (ARA-C) Event-free survival
Figure 2:Italian childhood APL. Treatment history
Table 1:Characteristics and results of children enrolled in different cooperative APL protocols (years 2001-2010
| Year | 2001 | 2004 | 2006 | 2006 | 2005 |
|---|---|---|---|---|---|
| Group | G-A-S* | France | PETHEMA** | CALGB*** | AIEOP/GIMEMAç |
| N. Patients | 81 | 31 | 66 | 57 | 124 |
| Induction therapy | ATRA^+ADE/AIE | ATRA+AD | ATRA+IDA | ATRA+DNR+CA | ATRA+IDA |
| ATRA dose/day | 25 mg/m2 | 45 mg/m2 | 25 mg/m2 | 25 mg/m2 | 25 mg/m2 |
| CR§ (%) | 95 | 97 | 92 | - | 96 |
| ID° (%) | 5 | 3 | 7 | - | 4 |
| Resist. (%) | 0 | 0 | 0 | 0 | 0 |
| 5-year EFS | 76 | 71 | 77 | 62 | 76 |
| 5-year OS | 87 | 90 | 87 | 86 | 89 |
| Cum. Anthracycline dose/m2 | DNR°° 60; IDA^^ 24; ADR^^^ 120 | DNR 495 | IDA 80; MTZ§§ 50/100 | IDA 80; MTZ§§ 50 |
*G-A-S=Germany-Austria-Switzerland;** PETHEMA=Programa Español de Tratamientos en Hematología; ***CALGB=Cancer and Leukemia Group B; çAIEOP/GIMEMA=Italian Pediatric Hematology-Oncology Group/Italian Adult Hematology Group; §CR=complete remission; °ID=induction death; ^ATRA=all-trans retinoic acid; °°DNR=daunorubicin;^^IDA=idarubicin; ^^^ADR=adriamycin; §§MTZ=mitoxantrone
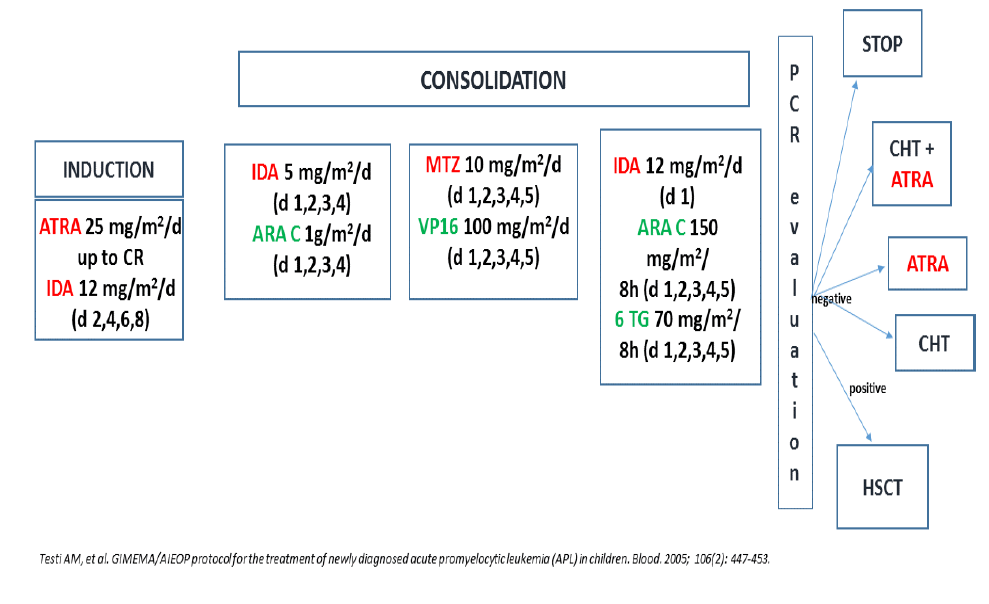
Figure 3:GIMEMA/AIEOP AIDA 0493 trial. Protocol design
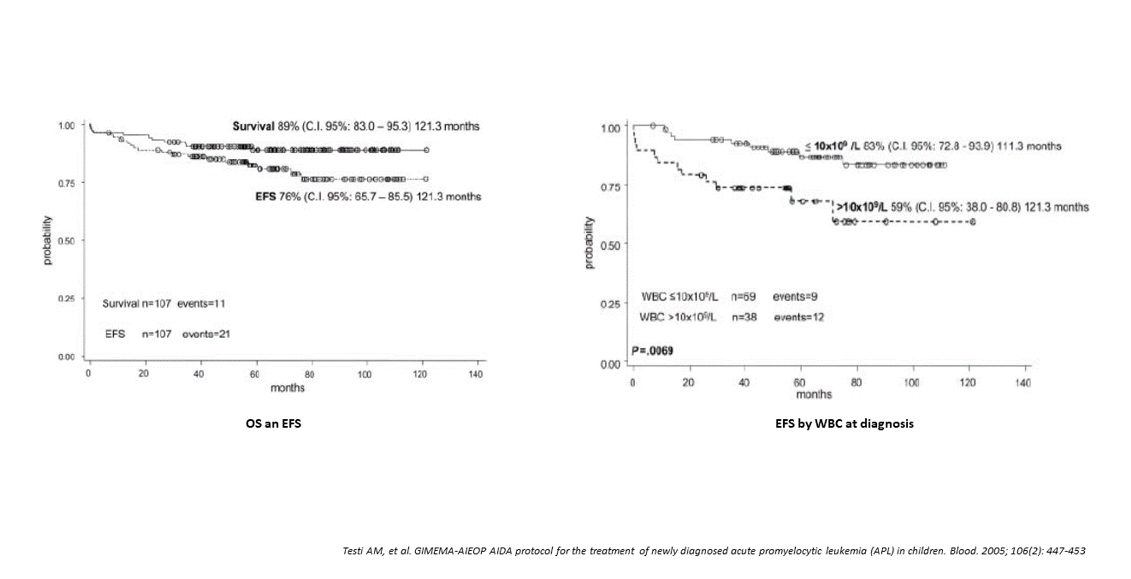
Figure 4:AIDA 0493 Protocol. Overall survival and event-free survival
Table 2:GIMEMA/AIEOP AIDA and ICC APL 01 trials: baseline features of children enrolled in the three consecutive protocols
| AIDA0493 | AIDA2000 | ICC APL 01 | p value | |
|---|---|---|---|---|
| N. pts* 110 | N. pts 127 | N. pts 258 | ||
| Follow-up; median (range) | 12.1 (0.03-16.3) | 12.9 (7.8-17.0) | 4.4 (0.1 – 9.5) | |
| Gender: M^/F^^ | 55/55 | 77/50 | 121/137 | ns** |
| Age years; median (min-max) | 11.6 (1.4-17.97) | 11.9 (1.1-17.98) | 10.3 (1.1-20.7) | ns |
| WBC°-x109/L; median (min-max) | 3.95 (0.3-180.0) | 3.60 (0.20-187.0) | 6.3 (0.08-339.0) | ns |
| Plts°°-x109/L; median (min-max) | 20.0 (3.0-48.0) | 27.5 (7.0-205.0) | 23.0 (2.0-262.0) | ns |
| FAB type: M3/M3v/na | 98/12 | 105/22 | 210/44/4 | ns |
| PML/RARA isofor Bcr: 1/2/3/nk | 57/5/36/12 | 50/6/37/34 | 110/7/102/39 | ns |
| Risk group: SR§/HR§§ (%) | 72 (65)/38 (35) | 85 (67)/42 (33) | 149 (58)/109 (42) | ns |
*pts=patients; ^M=male; ^^F=female; **ns=not significant; °WBC=white blood cell; °°Plts=platelets; §SR=standard-risk; §§HR= high-risk
Table 3:GIMEMA/AIEOP AIDA and ICC APL 01 trials: induction results of the three consecutive protocols
| Trial | AIDA0493 | AIDA2000 | ICC APL 01 |
|---|---|---|---|
| Evaluable pts*. | 107 | 125 | 250 (97) |
| HCR**, n. (%) | 103 (96) | 121 (97) | 250 (97) |
| Early death, n. (%) Risk category |
4 (4) 4 HR° |
4 (3) 4 HR° |
8 (3) 1 SR°°/ 7 HR |
| Cause of death | 3 ICH^; 1 sepsis | 3 ICH^; 1 sepsis | 8 ICH |
| N. resistant pts | 0 | 0 | 0 |
*pts=patients; **HCR=hematologic complete remission; °HR=high-risk; °°SR=standard-risk; ^ICH=intracranial hemorrhage
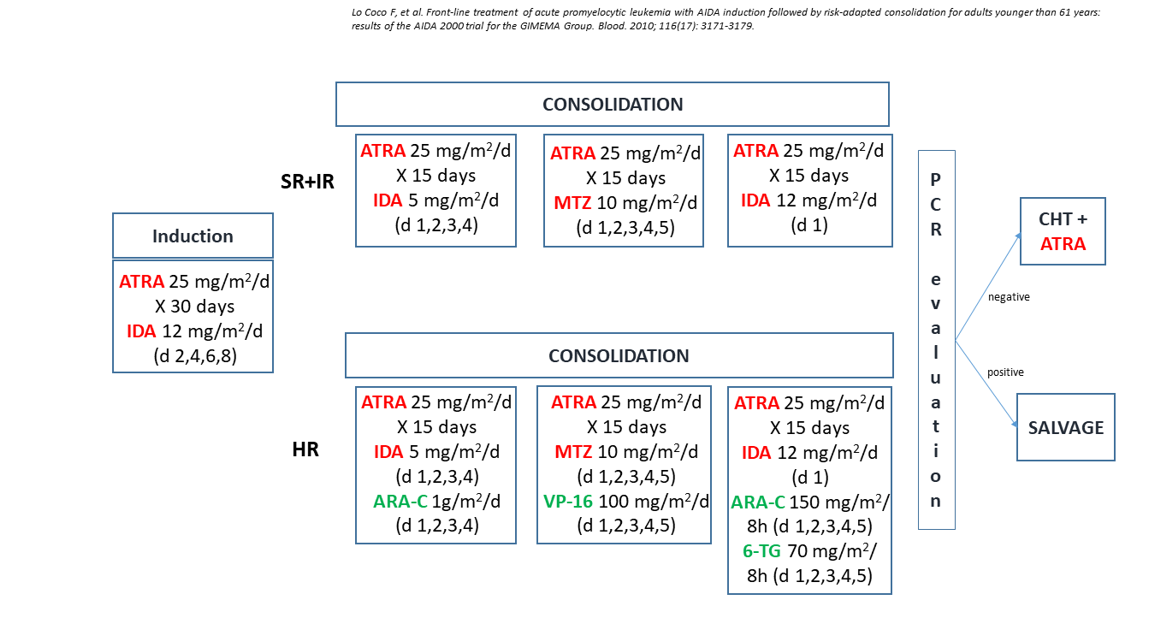
Figure 5:GIMEMA/AIEOP AIDA 2000 trial. Protocol design
At that time, this study represented the largest pediatric APL cohort homogeneously diagnosed and treated with a specific disease-tailored protocol. The induction combination of ATRA+idarubicin proved highly effective with no patient resistant to treatment.
In an attempt to reduce the ATRA-related neurotoxicity, more frequently observed in children and adolescents, in this protocol, the daily dosage of ATRA was decreased from 45 mg/m2/day to 25 mg/m2/day, a dose that had proven effective in a previous adult APL dose-reduction trial [25]. In spite of such reduction, both headache and pseudotumor cerebri were observed in 14 and 10 children, respectively, while an overt DS occurred only in 2 cases. All side effects were transient, reversible, and never cause of death. Febrile neutropenia, infections and mucositis were frequently observed during consolidation; no deaths in remission were recorded but treatment was withdrawn in 6 children. Therefore, consolidation toxicity was less severe in children compared to adults. Among the 229 adults treated with the AIDA0493 protocol and in HCR after induction, 5 died of complications and 16 did not complete the consolidation because of therapy-related toxicity [24]. The role of ATRA in the post-remission phase was, at that time, still controversial. The combination of intermittent maintenance ATRA+chemotherapy appeared to be particularly useful for patients presenting with a high WBC count. With regard to maintenance, this study allowed to establish an advantage in terms of improved “molecular” DFS for patients receiving ATRA+chemotherapy compared to those treated with ATRA alone [24].
At that time, other four studies have reported therapeutic results in childhood APL using combinations of ATRA and different chemotherapeutic drugs (Table 1). The first, from the German-Austrian-Swiss group, included 42 children with APL who received ATRA as an addition element of the AML-BFM-93 induction therapy and were retrospectively compared to other 41 children who received conventional therapy based on the AML-BFM-93 protocol [26]. A HCR was achieved in 95% of patients in the ATRA group; one boy died early due to hemorrhage. In the control group, 7 ED occurred. With a median time of 2.8 years, the 5-year EFS and OS of children treated with ATRA followed by chemotherapy were significantly better compared to conventionally treated children, (OS 87% versus 45% and EFS 76% versus 43%) (Table 1). The European APL 93 trial treated children with ATRA followed or combined with daunorubicin-cytarabine and then randomly assigned between no maintenance and intermittent ATRA, continuous chemotherapy or both [2]. Of the 576 patients enrolled, 31 were children; the HCR rate was 97%. In pediatric age, the 5-year OS, EFS and relapse rates were 90%, 71% and 27%, respectively; none of the 8 patients who received ATRA+chemotherapy as maintenance relapsed (Table 1). The authors concluded that ATRA combined with chemotherapy for induction and maintenance provided results in children with APL as favorable as in adults and constituted, at that time, the reference first-line treatment in both age groups.
Sixty-six consecutive children <18 years with newly diagnosed APL were enrolled in two sequential Spanish Programa para el Estudio y Tratamiento de las Hemopatias Malignas (PETHEMA) trials (simultaneous combination of ATRA+idarubicin). Treatment was identical to that used in adults except for a reduction of the ATRA dose, from 45 to 25 mg/m2/day, in all therapeutic phases. Consolidation consisted of three courses of anthracycline monochemotherapy [1]. Patients with an initial WBC≥10x109/L received consolidation with ATRA and a reinforced dose of idarubicin. Maintenance consisted of ATRA, low-dose mercaptopurine and methotrexate for all patients. A HCR was achieved in 92% of patients; the 5-year cumulative incidence of relapse (CIR) was 27%, whereas the OS and EFS rates were 87% and 77%, respectively (Table 1). Finally, CALGB C9710 treated adult and pediatric APL patients with ATRA and chemotherapy-based induction (daunorubicin+cytarabine) [27]. Fifty-seven children (age <15 years) were enrolled in this study; the 3-year OS and EFS were 86% and 62%, respectively (Table 1).
Take home messages
• The induction combination of ATRA plus idarubicin is highly effective with no patient showing evidence of resistance to treatment.
• In the pediatric age, ATRA is equally effective and less toxic at a dose of 25 mg/m2/day.
• The presenting WBC count significantly affects patients’ outcome.
• The combination of intermittent maintenance ATRA and chemotherapy appears to be particularly useful for HR patients.
Risk-Adapted First-Line Therapy
In 2000, a joint meta-analysis of the PETHEMA and the GIMEMA groups was carried out to identify relapse risk criteria in APL patients who had received an identical AIDA (ATRA+idarubicin) induction and a similar anthracycline-containing chemotherapy consolidation [14]. The resulting prognostic score (Sanz risk-score) that segregated discrete relapse risk groups based on the initial leukocyte and platelet counts, led to the design of two risk-adapted trials in which consolidation varied according to risk category. Using increased anthracycline doses in the HR group, the PETHEMA reported an improved outcome in this category as well as an overall improvement of results in all groups [14].
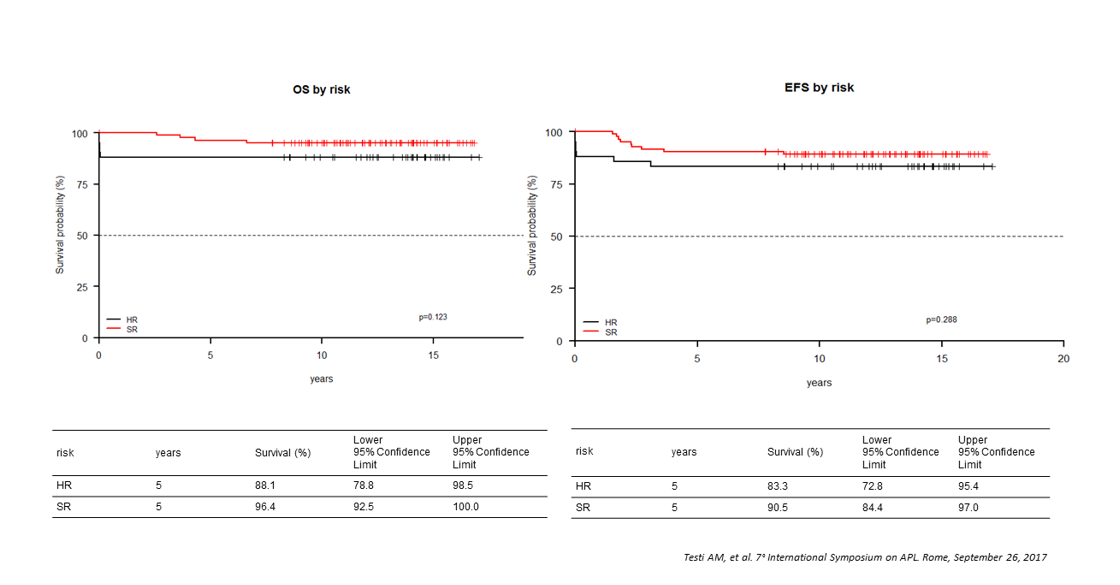
Figure 6:AIDA 2000 protocol. Overall survival and event-free survival by risk group
The GIMEMA risk-adapted trial (AIDA2000), that enrolled adults and children with newly diagnosed APL, was designed with the aim of investigating the effects on patients’ outcome of two main modifications from the original AIDA0493 protocol: the omission of cytarabine in the low/intermediate risk groups and the addition of ATRA during consolidation for all risk categories (Figure 5) [28,29].
Both AIDA2000 and the previous AIDA0493 treatment protocols combined ATRA and idarubicin in induction and delivered anthracycline-based consolidation. In AIDA2000, consolidation was based on the Sanz risk-score [14] and cytarabine, etoposide and thioguanine were omitted for non-HR patients. The results, analyzed separately for adults and children, showed OS rates of 80% in both age groups [28,29]. In children, the AIDA2000 study initiated in June 2000 and closed in January 2009. Relapse-risk groups were defined as follows: SR patients had a WBC count<10x109/L and HR a WBC count10x109/L, at diagnosis. A total of 127 children were eligible. The main diagnostic clinical and biologic characteristics of these patients were comparable with those included in the previous AIDA0493 (Table 2). A HCR was achieved in 96% of children; three ED (intracranial hemorrhage-ICH), occurred during induction; these children presented laboratory signs of coagulopathy at disease presentation. Another child died from severe infection (induction day 20). All children with induction death were HR (median WBC 65x109/L); no difference in the induction death rate was recorded in the two protocols (4% in both) (Table 3). In both protocols, no primary resistance to therapy was observed. ATRA was administered for a median of 28 (range 1-30) and 32 days (range 1-56) in the AIDA2000 and AIDA0493 trials, respectively [3,28]. Univariate and multivariate analysis for induction response showed that the presenting WBC count was an unfavorable factor for HCR achievement (p<.01). Univariate and multivariate analyses demonstrated no significant relationship between the other diagnostic clinico-biological parameters, such as platelet count (< and ≥40x109/L), FAB subtype (M3 or M3 variant) and PML-RARA transcript type [3,28].
In the AIDA2000 protocol, all children completed the three consolidation courses compared to 6 patients who had to discontinue therapy in the previous AIDA0493 trial.
RT-PCR test for PML-RARA was performed in 121 children after consolidation; molecular persistence of PML-RARA rearrangement was detected in 3/121 cases (2.5%) compared to 3/94 (3.2%) in the previous AIDA0493 [3,28] (Table 4). With AIDA2000, all children received ATRA containing maintenance. The 5-year OS and EFS were 93.7% and 84.9% in AIDA2000 compared to 89% and 76% in the previous AIDA0493 trial (Figures 4 and 6).
Induction therapy using concomitant ATRA and single agent chemotherapy with idarubicin confirmed its high efficacy (HCR 96% in both trials; absence of resistant disease). The induction death rate in the two protocols was similar (3% vs 4%). In agreement with what reported in other APL trials where ATRA was associated with one or more chemotherapeutic agents in induction, a higher presenting WBC count was an unfavorable factor for induction response [4,11,29,30].
In an international retrospective study including 608 children with newly diagnosed APL (236 Italian pediatric patients), the initial WBC count was significantly associated with ED [11].
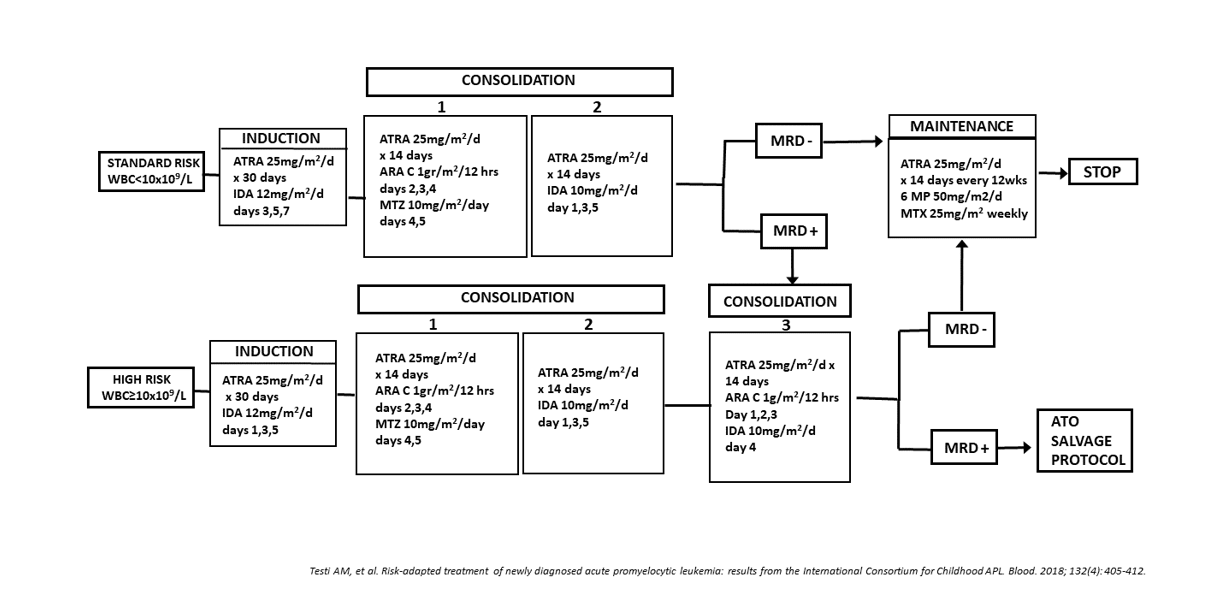
Figure 7:ICC APL 01 trial. Protocol design
The AIDA2000 trial with a risk-adapted consolidation strategy, resulted in a significant outcome improvement in children with APL. As reported for adults [29], also in pediatric age, non-intercalating agents can be safety removed from the front-line treatment of non-HR APL patients, reducing the toxicity in this subset of patients. No children treated in the AIDA2000 trial had to discontinue consolidation for therapy-related toxicities; the addition of ATRA during consolidation compensated for the less intensive chemotherapy in SR patients (EFS 90.5% and 83% in AIDA2000 and 0493, respectively) (Figures 4 and 6). The benefit of prolonged ATRA in consolidation was also observed in HR patients (5-year EFS was 83.3% vs 59% for AIDA0493 trial) (Figures 4 and 6) [3,28]. The improved results obtained in the HR group compared favorably with those achieved in adults (younger than 61 years) treated with the same AIDA2000 regimen [29]; the 6-year DFS in these HR patients was 84.5% in AIDA2000 compared to 49.6% of the AIDA0493 trial. These results in Italian children also confirmed those observed in 81 pediatric APL patients treated with an anthracycline-cytarabine regimen (AML-BFM trials) in combination with extended ATRA [31].
Take home messages
• Induction therapy with ATRA and idarubicin has confirmed its very high efficacy with a virtual absence of resistant disease in pediatric patients.
• Relapse risk groups based on the initial leukocyte count have led to the design of risk-adapted trials in which consolidation varied according to the risk category.
• ATRA extended consolidation has improved results in all risk categories.
ATRA and Decreased Anthracycline Cumulative Dose. First-Line Treatment for Childhood and Adolescent APL
The AIDA2000 data in pediatric and adult patients are in agreement with those from several other adult studies, including the successive risk-adapted LPA99 and LPA2005 trials, the French-Spanish meta-analysis and the randomized MRC-PETHEMA trial [14,32-34]. Taken together, the results of these studies indicated that with inclusion of ATRA during consolidation, cytarabine could be safely omitted from the front-line treatment of non-HR APL patients, allowing a significant spare of toxicity in this patient subset.
However, the anthracycline cumulative dose was very high (650 mg/m2 of anthracycline daunorubicin-equivalence) in the Italian APL trials and associated with a significant risk of both acute and long-term sequelae (e.g. cardiomyopathy and second neoplasms), a significant obstacle towards the achievement of a long-term favorable outcome, especially in children with a long-life expectancy. Based on these considerations, a new study (ICC-APL-01 trial) was conducted in the framework of the International Consortium for Childhood APL for the treatment of children/adolescents with newly diagnosed APL. The ICC-APL-01 trial was designed with the aim of reducing the cumulative anthracycline doses while maintaining an excellent likelihood of cure and of investigating the effects on patients’ outcome of a risk-adapted consolidation based on the initial WBC count (Figure 7). The study recruited 258 children/adolescents with APL. Patients were stratified into SR and HR groups according to baseline WBC counts (<10x109/L or ≥10x109/L); both groups received an identical induction treatment with ATRA+idarubicin. Two or three blocks of consolidation were administered to SR and HR patients, respectively, while maintenance with low dose chemotherapy+ATRA was given to all patients for 2 years. The cumulative dose of anthracyclines daunorubicin-equivalent in SR and HR patients was lower than that of previous studies (355 mg/m2 and 405 mg/m2, respectively). Patients’ clinical and biological characteristics (Table 2) were compared with those of children enrolled in the two previous Italian trials. ICC-APL-01 patients had a higher WBC count (6.3, 3.6 and 3.95x109/L, respectively) and consequently the percentage of HR patients was slightly higher in the last protocol (42%, 33%, 35%). Two-hundred and fifty children (97%) achieved a HCR (Table 3); 8 died from ICH. These patients presented with initial laboratory/clinical coagulopathy signs; the median WBC count was 73x109/L (range 1.8-257.0) and only 1 belonged to the SR group. No differences were recorded compared to the previous AIDA protocols, in terms of induction responses (HCR 97%, 97% and 96%) and deaths (3%, 3% and 4%) (Table 3). Of the 250 HCR patients (148 SR, 102 HR) who proceeded to consolidation, 218 were evaluable for molecular response after consolidation: 117/125 (94%) SR patients achieved a molecular CR (mCR) after the second consolidation cycle; 8, still PML-RARA+, received the third course and 5 achieved a mCR. Of the 93 HR patients, 91 (98%) tested PCR- after the third consolidation course. A molecular persistence of the PML-RARA rearrangement was detected in 5 patients (3 SR, 2 HR) (2.3%). No difference in molecular response was observed among the three consecutive trials [28] (Table 4).
Table 4:GIMEMA/AIEOP AIDA and ICC APL 01 trials: PML/RARA results after 3rd consolidation course
| PCR* | AIDA0493 | AIDA2000 | ICC APL 01 |
|---|---|---|---|
| N. pts° 95 | N. pts 121 | N. pts 218 | |
| Negative (%) | 92 (96.8) | 118 (97.5) | 213 (97.7) |
| Positive (%) | 3 (3.16) | 3 (2.48) | 5 (2.3) |
PCR=polymerase chain reaction; °pts=patients
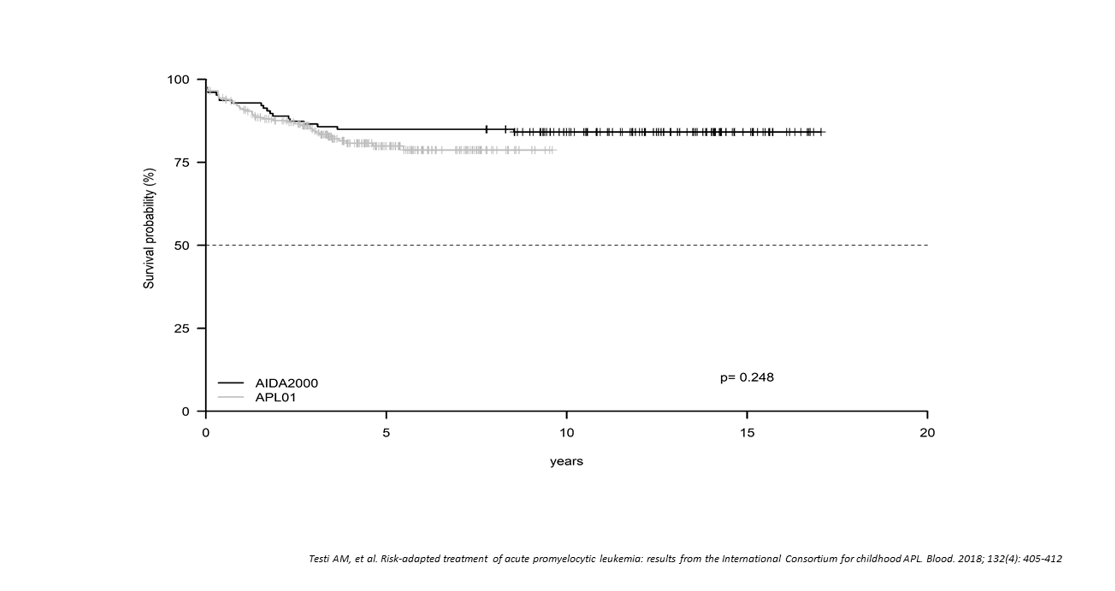
Figure 8:ICC APL 01 and AIDA 2000 protocols. Event-free survival
The 5-year OS and EFS for all patients enrolled in the ICC-APL-01 protocol were 94.6% and 79.9%, respectively. These results were comparable to those obtained in the AIDA2000 protocol (OS and EFS, 93.7% and 84.9%; p=.704 and p=.248, respectively) (Figure 8). SR had a better OS and EFS than HR patients in the ICC-APL-01 study. The OS was 98.4% and 89.4%, and the EFS was 84.3% and 74.2%, respectively, for SR and HR patients (p=.002 and p=.043). The outcome of SR and HR patients treated with the ICC-APL-01 protocol did not differ significantly from that of patients enrolled in the AIDA2000 trial (SR p=.40 and p=.37; HR p=.77 and p=.68 for OS and EFS, respectively). Risk-adapted consolidation strategies, with ATRA extended to consolidation and maintenance, resulted in an excellent outcome in SR children in the ICC-APL-01 and AIDA2000 protocols (OS 98.4% and 96.4%; EFS, 89.4% and 88.1%, respectively). The outcome of the ICC-APL-01 SR patients with reduced cumulative anthracycline dose (355 mg/m2) was not inferior to that reported in other APL studies employing significantly higher anthracycline doses (AIDA0493: SR OS 96%; PETHEMA/LPA-2005: SR OS 88%). These results were confirmed in 81 pediatric APL patients treated with an anthracycline-cytarabine regimen in combination with extended ATRA [31].
The benefit of prolonged ATRA in consolidation was also observed in HR patients. OS and EFS for these patients were 89.4% and 74.2% in ICC-APL-01, and 88.1% and 78.6% in AIDA2000; these values were superior to those achieved in the historical comparator AIDA0493 trial (OS 81%; EFS 59%) [3]. The HR group results are comparable to those obtained in adult patients (<61 years) treated with the AIDA2000 protocol (6-year OS in HR patients 87.4%) [29]. These data confirmed that limiting the anthracycline cumulative dose does not compromise the outcome of childhood APL.
The contribution of cytarabine in improving outcome has remained controversial, as some studies showed a superior outcome with cytarabine in induction whereas others did not [35,36]. Cytarabine increases the risk of prolonged myelosuppression and subsequent infectious complications. Prolonged maintenance with oral chemotherapy had also been part of the treatment of APL. Whether maintenance is required to maintain the current outcome remains a matter of debate. A Cochrane review analyzed data from 10 different trials and over 2000 patients of varying ages with or without the use of maintenance [37]. Treatment regimens varied widely among studies, as some used ATRA alone, some used chemotherapy alone and some used combined therapy. The data showed no significant improvement in OS with or without maintenance or among different types of maintenance regimens [37].
Take home messages
• Reduced cumulative anthracycline dose is associated with responses not inferior to those observed with significantly higher doses.
• Whether or not maintenance treatment with oral chemotherapy and intermittent ATRA is required is still debated.
What have We Learned. Role and Impact of Minimal Residual Disease (MRD) Monitoring
MRD monitoring based on the detection of PML-RARA transcripts employing the PCR technology has clearly demonstrated a benefit in the follow-up of APL patients. Following early ATRA introduction in APL therapeutic schemes, several retrospective and prospective studies were performed in large cohort of patients with the aim of better assessing the prognostic value of longitudinal PCR testing. The first studies showed that ATRA alone was associated with persistence of the PML-RARA transcript and subsequent disease relapse. By contrast, ATRA combined with chemotherapy induces a mCR in the majority of patients, which translates into a high probability of long-term remission and cure. In these studies, PML-RARA positivity was frequently detected at the end of the induction but was not predictive of relapse, being most probably related to a slower maturation kinetic under the action of ATRA. In the AIDA0493 and the UK-based MRC trials, 163 and 239 pediatric and adult patients were monitored adopting a PCR assay with a sensitivity of 10-4; at recovery from induction, 40-64% of patients in the AIDA and MRC trials, respectively, tested PCR-positive. In both studies most of these patients converted to a PCR-negative at the end of consolidation [38,39]. This time point is considered a therapeutic objective for patients with APL able to influence the outcome and the subsequent therapeutic decisions. The relapse risk was 57% versus 27% for positive patients compared to those who proved negative. The PCR positivity at the end of consolidation was highly predictive of hematological relapse, while a PCR negativity is strongly associated with long-term remission.
The above studies provided also a rationale for the administration of pre-emptive therapy in patients experiencing a molecular relapse. In both studies, patients who converted from a PCR- to a PCR+ status, confirmed with a new marrow sample within 2 weeks, during the follow-up underwent hematological relapse at a median of 3 months from PCR-positivity. These studies contributed to the recommendations of serial MRD monitoring in the European LeukemiaNet (ELN) disease guidelines [40].
Although RT-PCR assays have proved highly informative, they lack the capacity to distinguish between rising and falling numbers of fusion transcripts. Importantly, they also cannot reliably identify poor quality samples that could potentially give rise to false-negative results. These problems have been improved with the advent of real-time-quantitative PCR (RQ-PCR) which allows a better identification of poor quality samples, facilitating the absolute quantification of leukemic targets [41].
In a randomized study, RQ-PCR analysis for PML-RARA after induction showed that the proportion of patients with detectable transcript was higher in the ATO+ATRA than in the ATRA+chemotherapy arm (76% vs 63%), clearly reflecting a delayed maturation of leukemic cells rather than resistance [43]. This finding is not indicative of treatment failure and does not justify any treatment modification. The median time to CR using ATRA+chemotherapy is 4-5 weeks; however, a proportion of patients requires continuation therapy up to 8-10 weeks.
Keeping in mind the virtual absence of disease resistance and the frequent misleading persistence of late maturing blasts at post-induction morphologic assessment, as well as the lack of important prognostic factors at this point, the indication for marrow assessment after induction is questionable, except for research purposes. The achievement of a mCR at the end of consolidation has confirmed to be the major treatment objective in the management of APL.
The kinetics of initial treatment response were found to be an independent prognostic factor; patients with transcripts still detectable following the second course of therapy (who accounted for 23% of cases) were at a significantly increased risk of relapse (3-year relapse risk 19% vs 8%; p=.003). In the ICC APL 01 protocol, patients with an initial WBC count<10x109/L were monitored by RQ-PCR at recovery from the second consolidation course and if they remained MRD+ they were shifted to HR treatment. MRD assessment after the third consolidation course provided a much more powerful predictor of relapse than the presenting WBC count, widely used to guide risk-stratified treatment [42].
A longitudinal comparative RQ-PCR study of paired bone marrow and peripheral blood samples for PML-RARA monitoring showed an earlier detection of molecular relapse in the bone marrow. These data suggested that bone marrow sampling is the preferred approach [42]. Nevertheless, monitoring in peripheral blood remains a reasonable and more comfortable option, especially for pediatric patients.
Take home messages
• RT-PCR assays provided highly informative in APL patients, but cannot identify poor quality of marrow samples and lack the capacity of distinguish between rising and falling numbers of fusion transcript
• RQ-PCR assays enable a better identification of poor quality samples and facilitate the absolute quantification of leukemic targets
• PML/RARA positivity at the end of induction is not predictive of relapse, being related to a slower maturation kinetics under the action of differentiating agents
• MRD assessment at the end of consolidation is the major treatment objective in APL and provides more powerful predictor of relapse than presenting WBC
First-Line Treatment of Pediatric APL. Improved Outcome with ATO
The clinical use of ATO has been an additional step in the successful story of APL treatment and has further challenged the need for conventional chemotherapy in the management of APL. ATO was introduced in the 1990s primarily as a relapse therapy given as a single-agent [44]. ATO triggers apoptosis in APL cells and maturation in promyelocytes, working synergistically with ATRA to induce differentiation [45]. Following the success of ATO in relapse, it was trialed in front-line therapy in combination with ATRA, though it was initially limited to post-induction courses. In these studies, mostly conducted in adults, the combination of ATO/ATRA during consolidation was associated with significantly less myelosuppression and fewer infections. The COG-AAML0631 front-line protocol, that included 101 children, combined ATO and low-dose anthracycline for consolidation of SR and HR patients [13]. This trial demonstrated that ATO consolidation allowed a significant reduction in anthracycline doses while maintaining excellent results (3-year OS and EFS 94% and 91%, respectively; 3-year relapse incidence 4%) [13]. The North American APL Intergroup study CALGB 9710 randomized 28 patients, aged 15-18 years, to two consolidation courses with ATO (16 patients) and standard consolidation (12 patients) after a classic ATRA/chemotherapy-based induction. The 5-year OS was similar between the two groups (100% and 92% no-ATO and ATO, respectively), but the 5-year EFS was 56% for those no-ATO compared with 92% for those patients treated with ATO, with a lower relapse rate in these last patients (0% vs 44%) [46].
Recent results suggest that the ATO/ATRA combination may allow highly effective treatment without systemic chemotherapy in SR patients, while being associated with a more manageable toxicity. The APML4 trial by the Australasian Leukaemia and Lymphoma Group (ALL-G) was the first large clinical trial to include ATO/ATRA in induction and enrolled adult and pediatric patients [47]. This study removed anthracyclines for SR and limited anthracycline for HR patients only to induction. The combined use of ATO/ATRA in induction and post-induction therapy achieved EFS and OS rates of 92% and 96%, respectively, for SR and 83% and 87% for HR patients with a CIR of 5% in all groups [8]. The AML17 trial included all risk groups with newly diagnosed APL and compared the chemotherapy-free ATO+ATRA regimen with the standard ATRA-chemotherapy (ATRA+idarubicin). The CR rates were comparable between the two groups, but the ATO+ATRA group was associated with a better 4-year EFS (91% vs 70%, p=.002) [48]. In newly diagnosed non-high risk APL adult patients, a randomized multi-center trial (APL0406) conducted in Italy and Germany, in 2013, compared the ATO+ATRA combination for induction and consolidation with ATRA+idarubicin (AIDA) therapy. Similar CR rates were observed (100% vs 95%); the EFS at 50 months for patients in the ATO+ATRA versus ATRA+chemotherapy arms were 97.3% and 80% [49,50].
The combination of two differentiating drugs without chemotherapy might lead to an increased risk of leukocytosis and DS. It has been reported that 35%-47% of non-HR adults develop leukocytosis during chemotherapy-free induction; chemotherapy could be necessary to control leukocytosis and the oral administration of hydroxyurea (HU) is the most frequent utilized drug in the clinical trials.
The ATO/ATRA association is now the standard of care for SR adult APL, though most HR patients still receive anthracyclines during induction. More recently, the MD Anderson Cancer Center reported a CR rate of 96% and a 5-year EFS of 81% in HR patients treated with ATO, ATRA and gentuzumab-ozogamycin (GO) for induction and ATO+ATRA for consolidation [51]. Currently, there is no definitely recommended treatment option for HR patients.
The use of ATO in front-line treatment has further challenged the benefit of maintenance , as the results from the Italian-German APL0406 study demonstrated a 5-year OS of 99.2%, an EFS of 97% and a relapse rate of less than 2% in SR adults treated with ATRA and ATO alone [49,50]. The recent Children’s Oncology Group APL study, AAML1331, removed maintenance from both SR and HR treatment groups to validate these results in children.
Since 2004, several studies including ATO in the front-line treatment of childhood APL have been published. In the first experience [52], eleven children were treated with 8 ATO cycles (induction, consolidation, six maintenances), with a daily dosage of 0.15 mg/kg. Ten patients (91%) obtained a HCR (1 ED from ICH); 9 of them were RT-PCR- for PML-RARA at the end of consolidation. With a median follow-up of 30 months (range 3-60), the EFS and OS were 81% and 91%, respectively (Table 5) [52].
From January 1999 to December 2005, in Tianjin, 65 pediatric APL patients were included in three groups for induction therapy (G1, G2, G3): G1 ATRA alone (40 patients), G2 ATO alone (8 patients), G3 combination of ATRA/ATO (15 patients). The median dose of ATO was 0.24 mg/kg/day, administered for a median of 28 days (range 3-40). In the ATO group, 2 patients (8.7%) experienced an ED and 1 (4.3%) was moved to ATRA for an ATO-related edema. After achieving a HCR, at least two courses of antracycline-based consolidation and two years of maintenance were planned. No statistical differences in the 5-year EFS, DFS and OS were observed between G1 and G2+G3: the EFS was 76.2% and 86.2%, the DFS 80.2% and 94.4%, and the OS 91.8% and 91.3%, for G1 and G2+G3, respectively. This study demonstrated a comparable good outcome in children treated with ATRA, ATO or ATRA-ATO in induction (Table 5) [53]. The optimal dose of ATO during induction and post-remission therapy for pediatric APL was evaluated in a multicenter phase I and pharmacokinetic study that included 14 refractory APL children [54]. ATO at the dose of 0.15 mg/kg/day was identified as the recommended dose in this setting of patients.
Table 5:Characteristics and results in children with APL treated with ATO as front-line therapy
| Authors | N. Patients | Treatment phase | ATO dose/day | CR* (%) | ID° (%) | EFS | OS |
|---|---|---|---|---|---|---|---|
| George et al. (2004) | 11 | Induction, consolidation and maintenance | 0.15 mg/kg | 91% | 9% | 81% (med. FU: 30 mos**) | 91% (med. FU: 30 mos) |
| Zhang et al. (2008 | 23 | Induction | 0.24 mg/kg (median dose) | 91.3% | 8.7% | 86.2% (5 yrs°°) | 91.3% (5 yrs) |
| Wang et al. (2010) | 35 | Induction and maintenance | 0.15-0.17 mg/kg | 85.7% | 8.6% | 78.3%±6.9% (5 yrs) | 82.7%±7.2% (5 yrs) |
| Zhou et al. (2010) | 19 | Induction and consolidation | 0.20 mg/kg (4-6yrs) 0.16 mg/kg (>6 yrs) |
89.5% | 10.5% | 72.7% (5 yrs) | 83.9% (5 yrs) |
| Zhang et al. (2011) | 10 | Induction | 0.28 mg/kg (median dose) | 100% | 0% | 100% (5 yrs) | 100% (5 yrs) |
| Cheng et al. (2013) | 43 | Induction and consolidation | 0.16 mg/kg | 95.3% | 4.7% | 92.5%±4.2% (5 yrs) | 95.3%±3.2% (5 yrs) |
| Creutzig et al. (2016) | 11 | Induction | 0.15 mg/kg | 100% | 0% | 100% (med. FU: 2.4 yrs) | 100% (med. FU: 2.4 yrs) |
| Kutny et al. (2017) | 93 | Consolidation | 0.15 mg/kg | 81% | 3.9% | 94%±5% (3 yrs) | 91%±6%. (3 yrs) |
| Strocchio et al. (2018) | 18 | Induction and consolidation | 0.15 mg/kg | 100% | 0% | 100% (med. FU: 24 mos) | 100% (med. FU: 24 mos) |
| Kutny et al. (2018) | 16 | Induction and consolidation | 0.15 mg/kg | 93.8% | 6.2% | 92% (5 yrs) | 92% (5 yrs) |
| Zhang et al. (2018) | 66 | Induction and consolidation | 0.2 mg/kg(<6yrs) 0.15 mg/kg (≥6 yrs) |
100% | 0% | 97.3 ± 2.7% (med. FU: 36 mos) | 100% (med. FU: 36 mos) |
*CR=complete remission; °ID=induction death; **mos=months; °°yrs=years, ^FU=follow-up
In 2010, Wang described the experience in 35 newly diagnosed APL Chinese children, who received ATO, as monotherapy, in induction and maintenance [55]. In the attempt to avoid ATO-related hyperleukocytosis, cytarabine hydrocloryde (atangbaogan) with or without a natural alkaloid (harringtonine) was added. Nineteen children achieved a HCR with ATO monotherapy; 9 patients, who experienced a partial remission (PR), obtained a HCR after chemotherapy (CR rate 93.3%). This study confirmed the high CR rate, the long survival and low relapse rates (OS 82.7 ± 7.2%; 5-year EFS 78.3 ± 6.9%) of ATO treatment in pediatric APL (Table 5).
Another Chinese study included 19 children (age < 15 years) treated upfront with ATO alone during induction and post-remission therapy [45]. The ATO daily dose was 0.20 mg/kg in children ranging between 4 and 6 years, and 0.16 mg/kg in those older than 6 years. Seventeen patients obtained a HCR; 13 received post-remission therapy; 14 remained in first HCR (follow-up range 21-75 months), while 3 patients had a hematologic relapse. Two out of them obtained a second HCR with ATO monotherapy; with a median follow-up of 53 months (range 23-76), the estimated 5-year OS and EFS were 83.9% and 72.7%, respectively (Table 5) [45].
In 2011, Zhang and colleagues retrospectively reported the outcome of 27 pediatric patients with SR APL treated from January 1999 to December 2003. Seventeen (group-I) received ATRA alone, while 10 patients (group-II) because of ATRA-related side effects were switched to ATO alone or to ATO-ATRA, after ATRA dose reduction. The median dose of ATO in these patients was 0.28 mg/kg/day (range 0.18-0.38). No ED were seen in groups-I and -II, and all patients achieved a HCR after induction. The 5-year DFS was 74.2 ± 11.3% in group-I compared to 100% in group-II (p=.108) (Table 5). Four patients experienced a relapse in group-I compared to 0 in group-II (5-year CIR 25.8% and 0% in group-I and II, respectively). The conclusion of this study was that in SR pediatric APL patients the additional ATO in induction may decrease the relapse risk [56].
A front-line treatment with a combination of ATO/ATRA in both induction and consolidation was employed in 43 children and compared to 25 who did not received ATO [57]. However, the consolidation phase still contained anthracyclines and was followed by standard maintenance (chemotherapy+ATRA). The CR rate in the ATO-ATRA group was higher than in the ATRA-group (95.4% vs 80%); EFS, DFS and OS rates in the non-ATO were lower than in the ATO-group (70.4 ± 9.4%, 76.4 ± 9.2% and 70.4 ± 9.4% vs 92.5 ± 4.2%, 97.1 ± 2.9% and 95.3 ± 3.2%), confirming the advantage of adding ATO in the treatment of pediatric APL [57]. However, compared to adults, the incidence of leukocytosis in children, during ATO/ATRA induction, was higher, reaching more than 60% [36].
Following these encouraging results, ATO was introduced in the induction therapy of pediatric SR APL in Western countries. Eleven children with SR APL, treated with induction ATRA and AML-Berlin-Frankfurt-Munster-(BFM)-04 protocol, received ATO (0.15 mg/kg/day) at least 10 days after ATRA, to avoid hyperleukocytosis. All patients achieved a mCR after a median of 10 weeks (range 7-20) and all remained in remission at 2.4 years [58]. In the CCAPL2010 trial, 66 pediatric patients with newly diagnosed APL, received ATRA+ATO as induction followed by one course of idarubicin+ATO (28 days) and two courses with an anthracycline associated or not with cytarabine. All children achieved a HCR. With a median follow-up of 36 months, the EFS was 97.3% and the OS 100% [36]. In Italy, 18 newly diagnosed pediatric APL patients (16 SR, 2 HR) received the chemo-free protocol scheme described for adult SR APL [50]. All patients achieved a mCR after the third ATO consolidation course. With a median follow-up of 24 months (range 9-42), all patients were alive and in mCR (Table 5) [59].
The recent pediatric trials (North-American COG-AAML1331 and European ICC-APL-02) are testing a chemo-free approach (ATO+ATRA) for SR patients and ATO, ATRA and GO for those at HR. Such chemotherapy-free regimens are likely to reduce the risk of myelosuppression-related complications, cardiotoxicity associated with anthracycline and secondary malignancies described with the use of ATRA+traditional chemotherapy (incidence 1-3% in previous Italian studies) [3,28].
The striking outcome improvement obtained with ATO+ATRA regimens call into question the benefit of prolonged MRD monitoring, at least for non-HR patients. Although the recommendations from the ELN MRD Working Party [60] suggest the longitudinal monitoring of patients after consolidation (3-month interval for patients maintained with ATRA cycles), MRD monitoring may be omitted in non-HR patients treated with ATO+ATRA and achieving a mCR after consolidation.
In the pediatric age, prolonged arsenic exposure may lead to skin lesion (hyperpigmentation, keratosis), hypertension, diabetes and neurologic effects. Arsenic might cause an alteration of growth and development; however, available data are still immature to evaluate ATO long-term toxicities and we need to wait for the results of the ongoing trials.
Take home messages
• ATO has added to the treatment success story in APL, decreasing toxicity and improving the outcome.
• ATO/ATRA combination may allow highly effective treatment without systemic chemotherapy in SR patients and is associated with a more manageable toxicity.
• There is no definitely recommended treatment option for HR patients, though most of them receive anthracyclines or GO during induction.
ATO as Oral Formulation
ATO in the United States and in Europe is only approved in the intravenous formulations, requiring therefore prolonged hospital admissions or daily home or clinic infusions in order to receive treatment. Recently, ATO has been developed in an oral formulation that in international studies has shown a high bioavailability and similar effectiveness compared to the intravenous form [61,62].
In addition to ATO, another arsenic compound, As4S4, has recently proved highly effective in the treatment of adult APL. The Realgar-Indigo naturalis formula (RIF), a traditional Chinese medicine which can be taken orally, contains realgar (As4S4) as well as Indigo naturalis, Radix salviae miltiorrhizae and Radix pseudostellariae [63]. The components of RIF yield synergistic anti-leukemia effects in a murine APL model in vivo and on APL cell lines in vitro [63]. A Chinese multicenter clinical trial has confirmed the efficacy and safety of RIF in adults with APL [64]. More recently, a multicenter study conducted by the Chinese APL Cooperative Group demonstrated the non-inferiority of RIF compared to ATO when in combination with ATRA and chemotherapy in terms of efficacy and safety in adults [62]; the estimated 7-year EFS rates were similar between the two groups (93.7% vs 89.4%, P=.37) [65]. Moreover, oral RIF can be administered outside the hospital, reducing the number of hospital days compared to intravenous ATO [59,65]. In addition, oral ATO has fewer QTc prolonging side effects than the intravenous form. There is limited data on the role of RIF in the treatment of pediatric patients. Some small retrospective studies suggest that RIF is also safe and effective in children with APL, with less cardiac toxicity compared to ATO [66-68].
Studies utilizing oral ATO in pediatric patients are taking pace at international sites to determine if children achieve the same responses as in adults, as pharmacologic properties of medications can vary with age. With the availability of oral ATO, maintenance has become a realistic and convenient outpatient treatment. Four patients (age 3-11 years) with relapsed APL, were treated with oral ATO-based strategy without the use of HSCT [69]. ATO was administered as monotherapy or in combination with ATRA and was well tolerated.
The South China Children Leukemia Group (SCCLG) conducted a randomized study to compare the efficacy, safety and number of hospital days between RIF- and intravenous ATO-based therapies in pediatric APL [70]. Eligible patients, 16 years old or younger with newly diagnosed APL, were treated with a risk-adapted protocol. In particular, patients received oral ATRA at morphologic diagnosis with mitoxantrone (one or three doses in non-HR or HR patients, respectively). At genetically confirmed diagnosis, patients were randomly assigned to the ATO or RIF group. Patients in the ATO group, who achieved a HCR received three consolidation courses containing ATRA, ATO and low-intensity chemotherapy with mitoxantrone, and addition of cytarabine for HR. Patients assigned to the RIF group received the same treatment as those of the ATO group, but ATO was replaced by RIF. RIF was given orally at a dose of 135 mg/kg/day. From September 2011 to January 2017, 82 eligible patients were enrolled; 42 were assigned to the ATO and 40 to the RIF group. All 82 patients achieved a HCR; median time to HCR was 22.0 days (range 10.0-42.0) in the ATO and 24.5 days (range 14.0-46.0) in the RIF group (p=.168). Eighty-one patients (1 withdrawal), 42 in the ATO and 39 in the RIF group, achieved a mCR at the end of consolidation. With a median follow up of 3.0 years (range 0.5-6.4), the estimated 5-year OS and EFS rates were 100% in both groups. Leukocytosis, common in both groups, was successfully managed in all patients. Rates of infection, fever of unknown origin and headache were lower in the RIF than in the ATO group. The mean accumulated days required for inpatient management during induction and consolidation were 67.8-22.4 and 43.9-19.3 for non-HR patients in the ATO and RIF groups (p=.000), and 68.1-19.6 and 48.1- 18.6 for HR patients in the ATO and RIF groups (p=.029), respectively. RIF could be taken orally in an outpatient setting when the disease was stable, decreasing the risk of hospital cross-infections. There was no difficulty encountered with children taking RIF. In order to make RIF easier to be taken by young children, the pill was triturated in water and sugar was added if necessary [70]. Different kinetics of WBC proliferation have been observed during induction with oral arsenic+ATRA and intravenous ATO+ATRA. A higher WBC count was observed in the RIF group than in the ATO group after 10 days of treatment (9.22x109/L vs 4.0x109/L; p=.015) [71].
Take home messages
• ATO is only approved in the intravenous formulation and requires prolonged hospital admission
• RIF, another arsenic compound, can be orally administered and seems to be equally effective and to have fewer QTc prolonging side effects than the intravenous ATO form
• Limited data on the role of RIF in pediatric patients are available
Other drugs in APL
The addition of ATRA/ATO to the upfront treatment of APL has resulted in resistance to these treatments in the few patients who do relapse [72]. Resistance to ATRA is primarily due to mutations of the binding domain of RARA, demonstrated in 40% of relapsed adults previously exposed to ATRA therapy, thereby reducing the binding affinity of ATRA in these cases. The mechanism of resistance to ATO therapy is not well described. Current treatments at relapse include further ATO exposure, GO, an antibody-drug conjugate targeting CD33+ cells (highly expressed in APL). Other clinical trials utilize tamibarotene, a synthetic retinoid with higher binding affinity for PML-RARA still under investigation [73]. Unlike ATRA, the plasma level of tamibarotene does not decline after daily administration; these characteristics suggest that it could be clinically superior to ATRA. A phase 3 study comparing ATRA with tamibarotene as maintenance therapy for newly diagnosed APL patients (age 15-70 years) has been carried out by the Japan Adult Leukemia Study Group (JALSG). At a median follow-up of 7.3 years, maintenance therapy with tamibarotene was significantly superior to that of ATRA [73]. This new synthetic retinoid may lead to a new strategy for the management of HR APL patients.
Small molecule inhibitors with targets such as tyrosine kinases, telomerase and c-myc have demonstrated some efficacy in pre-clinical studies using APL cell lines but have so far not reached the clinical setting [74,75].
What it is still Unsolved in APL?
An open question is related to the mutation of fms-like tyrosine kinase 3 receptor-internal tandem duplication (FLT3-ITD), a genetic aberration frequently present in APL (30%-40% of cases) whose prognostic role is controversial. FLT3-ITD is more frequent in pediatric APL (40%) and is usually associated with a higher WBC count, a morphologic M3 variant and an involvement of the PML bcr-3 breakpoint [43]. The FLT3-ITD mutation does not seem to have an impact on both the EFS or the CIR in patients receiving ATO+ATRA, while in some reports a trend towards an inferior EFS has been observed in patients receiving ATRA+chemotherapy. In any case, the ATO+ATRA combinations seem to abrogate the potential negative prognostic impact of FLT3-ITD [76].
It has been reported that adult patients with CD56+ APL may have a higher early mortality rate compared to CD56- patients. In pediatric age, the incidence of CD56-positivity is around 10% of cases and is frequently associated with a presenting high WBC count and involvement of the PML bcr-3 breakpoint [77]. In the large study on the ED predictors in childhood APL, data of CD56 immunophenotyping were available in 228/683 children. Three of the 17 CD56+ patients (17.6%) had an ED, while 14/221 CD56- patients (6.5%) died early [11].
ED resulting from hemorrhage remains a major cause of induction failure. However, since the introduction of ATRA in induction and, more recently, with arsenic-based regimens ED have markedly reduced in both pediatric and adult studies. The interpretation of these findings has to take into account the inevitable selection of patients enrolled in the protocols, with some patients even failing to enter into clinical trials due to ED. Data from population-based studies, describing the “real-world” situation have reported much higher rates of ED (up to 29%) [78,79].
Conclusions
The advances in the management of APL both in children and adults have taken this form of AML from a disease with a notable morbidity and mortality to the one with the best outcome of all AML subtypes. This is largely due to the incorporation of ATRA and more recently of ATO in the front-line regimens. ATO is now routinely used with minimal or no chemotherapy, especially for SR patients.
ED before treatment or in the initial days remains the major obstacle towards the conclusive cure of APL. Efforts in this area should focus primarily on education programs aimed at improving the prompt suspect/diagnosis of APL and, consequently, a timely management of the disease. An earlier referral to specialized centers, no delay in the APL diagnostic work-up and in the ATRA/ATO administration, as well as optimization of the management of coagulation disorders, especially in HR patients, continue to be the necessary strategy to minimize ED in this disease.
Acknowledgments
The authors are grateful to all doctors dedicated to treatment of APL in children and adolescents. The authors wish to recall Prof. F. Lo Coco and Prof. E.H. Estey for their dedication to patients and intensive work for APL care.
Conflict of Interest
The authors have no conflicts of interest.
References
1. Ortega JJ, Madero L, Martin G, et al. Treatment with all-trans retinoic acid and anthracycline monochemotherapy for children with acute promyelocytic leukemia: a multicenter study by the PETHEMA group. J Clin Oncol. 2005; 23(30): 7632-7640. doi: 10.1200/JCO.2005.01.3359.
2. De Botton S, Coiteux V, Chevret S, et al. Outocme of childhood acute promyelocytic leukemia with all-trans retinoic acid and chemotherapy. J Clin Oncol. 2004; 22: 1404-1412. doi: 10.1200/JCO.2004.09.008
3. Testi AM, Biondi A, Lo Coco F, et al. GIMEMA-AIEOP AIDA protocol for the treatment of newly diagnosed acute promyelocytic leukemia (APL) in children. Blood. 2005; 106: 447-453. doi: 10.1182/blood-2004-05-1971
4. Testi AM, D’Angiò M, Locatelli F, Pession A, lo Coco F. Acute promyelocytic leukemia (APL): comparison between children and adults. Mediterr J Hematol Infect Dis. 2014; 6(1): e2014032. doi: 10.4084/MJHID.2014.032
5. Kudo K, Yoshida H, Kiyoi H, Numata S, Horibe K, Naoe T. Etoposide-related acute promyelocytic leukemia. Leukemia. 1998; 12: 1171-1175. doi: 10.1038/sj.leu.2401089.
6. Mazzarella L, Botteri E, Matthes A, et al. Obesity is a risk factor for acute promyelocytic leukemia: evidence from population and cross-sectional studies and correlation with FLT3 mutations and polyunsaturated fatty acid metabolism. Haematol. 2020; 105(6): 1559-1566. doi: 10.1016/j.critrevonc.2020.103187
7. Taga T, Tomizawa D, Takahashi H, et al. Acute myeloid leukemia in children: current status and future directions. Pediatr Int. 2016; 58: 71-80. doi: 10.1111/ped.12865
8. Iland HJ, Collins M, Bradstock K, et al. Use of arsenic trioxide in remission induction and consolidation therapy for acute promyelocytic leukemia in the Australasian Lekaemia and Lymphoma Group (ALLG) APML4 study: a non-randomised phase 2 trial. Lancet Haematol. 2015; 2: e357-366. doi: 10.1016/S2352-3026(15)00115-5
9. Rajpurkar M, Alonzo TA, Wang YC, et al. Risk markers for significant bleeding and thrombosis in pediatric acute promyelocytic leukemia; report from the Children’s Oncology Group Study AAML0631. J Pediatr Hematol Oncol. 2019; 41: 51-55. doi: 10.1097/MPH.0000000000001280
10. De Azevedo AC, Matsuda E, Cervellini JY, et al. Early mortality in children and adolescents with acute promyelocytic leukemia: experience of the Boldrini Children’s Center. J Pediatr Hematol Oncol. 2020; 42(7): e641-e646. doi: 10.1097/MPH.0000000000001601
11. Ala O, Ribeiro RC, Testi AM, et al. Predictors of thrombohemorragic early death in children and adolescents with t(15;17)-positive acute promyelocytic leukemia treated with ATRA and chemotherapy. Ann Hematol. 2017; 96: 1449-1456. doi: 10.1007/s00277-017-3042-6
12. Jin B, Zhang Y, Hou W, et al. Comparative analysis of causes and predictors of early death in elderly and young patients with acute promyelocytic leukemia treated with arsenic trioxide. J Cancer Res Clin Oncol. 2020; 46(2): 485-492. doi: 10.1007/s00432-019-03076-x.
13. Kutny MA, Alonzo TA, Gebing RB, et al. Arsenic trioxide consolidation allows anthracycline dose reduction for pediatric patients with acute promyelocytic leukemia: report from the Children’s Oncology Group Phase III historically controlled trial AAML0631. J Clin Oncol. 2017; 35: 3021-3029. doi: 10.1200/JCO.2016.71.6183
14. Sanz MA, Lo Coco F, Martin G, et al. definition of relapse risk and role of nonanthracycline drugs for consolidation in patients with acute promyelocytic leukemia: a joint study of the PETHEMA and GIMEMA cooperative groups. Blood. 2000; 96: 1247-1253.
15. Biondi A, Rovelli A, Cantù-Rainoldi A, et al. Acute promyelocytic leukemia in children: experience of the Italian Pediatric Hematology and Oncology Group (AIEOP). Leukemia. 1994; 8: 1264-1268.
16. Bernard J, Weil M, Boiron M, et al. Acute promyelocytic leukemia: results of treatment by daunorubicin. Blood. 1973; 41: 489-496.
17. Huang ME, ye YC, Chen SR, et al. Use of all-trans retinoic acid in the treatment of acute promyelocytic leukemia. Blood. 1988; 72: 567-572. doi: 10.1182/blood-2016-11-750182.
18. Wang ZY, Chen Z. Acute promyelocytic leukemia from highly fatal to highly curable disease. Blood. 2008; 111: 2505-2515. doi: 10.1182/blood-2007-07-102798.
19. Fenaux P, Le Deley MC, Castaigne S, et al. Effect of all trans-retinoic acid in newly diagnosed acute promyelocytic leukemia. Results of a multicenter randomized trial. European APL 91 Group. Blood. 1993; 82: 3241-3249.
20. Fenaux P, Castaigne C, Chevret S, et al. A randomized comparison of all trans-retinoic acid (ATRA) followed by chemotherapy and ATRA plus chemotherapy and the role of maintenance therapy in newly diagnosed acute promyelocytic leukemia. The European APL Group. Blood. 1999; 94: 1192-1200.
21. Tallman MS, Andersen JW, Schiffer CA, et al. All-trans retinoic acid in acute promyelocytic leukemia: long-term outcome and prognostic factor analysis from the North American Intergroup protocol. Blood. 2002; 100: 4298-4302. doi: 10.1182/blood-2002-02-0632.
22. Avvisati G, Mandelli F, Petti MC, et al. Idarubicin (4-demethoxydaunorubicin) as a single agent for remission induction of previously untreated acute promyelocytic leukemia: a pilot study of the Italian cooperative group GIMEMA. European J of Haematol. 1990; 44: 257-260. doi: 10.1111/j.1600-0609.1990.tb00389.x.
23. Avvisati G, Lo Coco F, Diverio D, et al. AIDA (all-trans retinoic acid + idarubicin) in newly diagnosed acute promyelocytic leukemia: a gruppo Italiano Malattie Ematologiche dell’Adulto (GIMEMA) pilot study. Blood. 1996; 88: 1390-1398.
24. Avvisati G, Lo Coco F, Paoloni FP, et al. GIMEMA, AIEOP, and EORTC Cooperative Groups. AIDA 0493 protocol for newly diagnosed acute promyelocytic leukemia: very long-term results and role of maintenance. Blood. 2011; 117: 4716-4725. doi: 10.1182/blood-2010-08-302950.
25. Castaigne S, Lefebvre P, Chomienne C, et al. Effectiveness and pharmacokinetics of low-dose all-trans retinoic acid (25 mg/m2) in acute promyelocytic leukemia. Blood. 1993; 82: 3560-3563.
26. Mann G, Reinhardt D, ritter J, et al. Treatment with all-trans retinoic acid in acute promyelocytic leukemia reduces early deaths in children. Ann Hematol. 2001; 80: 417-422. doi: 10.1007/s002770100304
27. Gregory J, Feusner J. Acute promyelocytic leukemia in childhood. Curr Oncol Rep. 2009; 11(6): 439-445. doi: 10.1007/s11912-009-0060-0
28. Testi AM, Pession A, Diverio D, et al. Risk-adapted treatment of acute promyelocytic leukemia: results from the International Consortium for Childhood APL. Blood. 2018; 132(4): 405-412. doi: 10.1182/blood-2018-03-836528.
29. Lo Coco F, Avvisati G, Vignetti M, et al. Italian GIMEMA Cooperative Group. Front-line treatment of acute promyelocytic leukemia with AIDA induction followed by risk-adapted consolidation for adults younger than 61 years: results of the AIDA-2000 trial of the GIMEMA Group. Blood. 2010; 116(17): 3171-3179. doi: 10.1182/blood-2010-03-276196
30. Ade’s L, Chevret S, Raffoux E, et al. European APL group. Long-term follow-up of European APL-2000 trial, evaluating the role of cytarabine combined with ATRA and Danorubicin in the treatment of nonelderly patients. Am J Hematol. 2013; 88(7): 556-559. doi: 10.1002/ajh.23451
31. Creutzig U, Zimmemann M, Dvorzak M, et al. Favourable outcome of patients with childhood acute promyelocytic leukemia after treatment with reduced cumulative anthracycline doses. Br J Haematol. 2010; 143(3): 399-409. doi: 10.1111/j.1365-2141.2010.08107.x.
32. Sanz MA, Montesinos P, Rayon C, et al. Risk-adapted treatment of acute promyelocytic leukemia based on all-trans retinoic acid and anthracycline with addition of cytarabine in consolidation for high-risk patients: further improvements in treatment outcome. Blood. 2010; 115: 5137-5146. doi: 10.3816/CLML.2010.s.025.
33. Sanz MA. Montesinos P, Kim HT, et al. IC-APL and PETHEMA and HOVON groups. All-trans retinoic acid with daunorubicin or idarubicin for risk-adapted treatment of acute promyelocytic leukemia: a matched-pair analysis of the PETHEMA LPA-2005 and IC-APL studies. Ann Hematol. 2015; 94(8): 1347-1356. doi: 10.1007/s00277-015-2393-0
34. Burnett AK, Hills RK, Grimwade D, et al. Inclusion of chemotherapy in addition to anthracycline in the treatment of acute promyelocytic leukaemia does not improve outcomes: results of the MRC AML15 trial. Leukemia. 2013; 27: 843-851. DOI: 10.1038/leu.2012.360
35. Zhang L, Zhu X, Chen X, et al. The role of standard-dose cytarabine in children with acute promyelocytic leukemia: A single-center experience. J Pediatr Hematol Oncol. 2011; 33: e46-50. doi: 10.1097/MPH.0b013e3181ed3384.
36. Zhang L, Zou Y, Chen Y, et al. Role of cytarabine in paediatric acute promyelocytic leukemia treated with the combination of all-trans retinoic acid and arsenic trioxide: a randomized controlled trial. BMC Cancer. 2018; 18: 374. doi: 10.1186/s12885-018-4280-2.
37. Muchtar E, Vidal L, Ram R, et al. The role of maintenance therapy in acute promyelocytic leukemia in the first complete remission. Cochrane database Syst Rev. 2013; 28(3): CD009594. doi: 10.1002/14651858.CD009594.pub2
38. Diverio D, Rossi V, Avvisati G, et al. Early detection of relapse by prospective reverse transcriptase-polymerase chain reaction analysis of PML/RARalpha fusion gene in patients with acute promyelocytic leukemia enrolled in the GIMEMA-AIEOP multicenter “AIDA” trial. GIMEMA-AIEOP Multicent. Blood. 1998; 92: 784-789.
39. Burnett AK, Grimwade D, Solomon E, et al. presenting white cell count and kinetics of molecular remission predict prognosis in acute promyelocytic leukemia treated with all-trans retinoic acid: results of the Randomized MRC trial. Blood. 1999; 93: 4131-4143.
40. Sanz MA, Fenaux P, Tallman MS, et al. Management of acute promyelocytic leukemia: updated recommendations from an expert panel of European LeukemiaNet. Blood. 2019; 133(15): 1630-1643. doi: 10.1182/blood-2019-01-894980.
41. Gabert J, Beillard E, van der Velden VH, et al. Standardization and quality control studies of “real-time” quantitative reverse transcriptase polymerase chain reaction of fusion gene transcript for residual disease detection in leukemia – a Europe against Cancer program. Leukemia. 2003; 17: 2318-2357. doi: 10.1038/sj.leu.2403135
42. Grimwade D, Jovanovic JV, Hills RK, et al. Prospective minimal residual disease monitoring to predict relapse of acute promyelocytic leukemia and to direct pre-emptive arsenic trioxide therapy. J Clin Oncol. 2009; 27: 3650-3658. doi: 10.1200/JCO.2008.20.1533.
43. Cicconi L, Divona M, Ciardi C, et al. PML-RARα kinetics and impact of FLT3-ITD mutations in newly diagnosed acute promyelocytic leukemia treated with ATRA and ATO or ATRA and chemotherapy. Leukemia. 2016; 30(10): 1987-1992. doi: 10.1038/leu.2016.122
44. Niu C, Yan H, Yu T, et al. Studies on treatment of acute promyelocytic leukemia with arsenic trioxide: Remission induction, follow-up, and molecular monitoring in 11 newly diagnosed and 47 relapsed acute promyelocytic leukemia patients. Blood. 1999; 34: 3315-3324.
45. Zhou J, Zhang Y, Li J, et al. Sigle-agent arsenic trioxide in the treatment of children with newly diagnosed acute promyelocytic leukemia. Blood. 2010; 115(9): 1697-1702. doi: 10.1182/blood-2009-07-230805.
46. Kutny MA, Geyer S, Laumann KM, et al. Outcome of pediatric acute promyelocytic leukemia patients at Children’s Oncology Group sites on the Leukemia Intergroup Study CALGB 9710 (alliance). Pediatr Blood Cancer. 2019; 66(3): e27542. doi: 10.1002/pbc.27542.
47. Iland HJ, Bradstock K, Supple SG, et al. All-trans retinoic acid, idarubicin and IV arsenic trioxide as initial therapy in acute promyelocytic leukemia (APML4). Blood. 2012; 120: 1570-1580. doi: 10.1182/blood-2012-02-410746
48. Burnett AK, Russell NH, Hills RK, et al. Arsenic trioxide and all-trans retinoic acid treatment for acute promyelocytic leukemia in all risk groups (AML17): results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2015; 16(13): 1295-1305. doi: 10.1016/S1470-2045(15)00193-X
49. Cicconi L, Platzbecker U, Avvisati G, et al. Long-term results of the all-trans retinoic acid and arsenic trioxide in non-high-risk acute promyelocytic leukemia: upadate of the APL0406 Italian-German randomized trial. Leukemia. 2020; 34(3): 914-918. doi: 10.1038/s41375-019-0589-3
50. Platzbecker U, Avvisati G, Cicconi L, et al. Impreved outcomes with retinoic acid and arsenic trioxide compared with retinoic acid and chemotherapy in non-high-risk acute promyelocytic leukemia: final results of the randomized Italian-German APL0406 trial. J Clin Oncol. 2017; 35: 605-612. doi: 10.1200/JCO.2016.67.1982.
51. Abaza Y, Kantarjian H, Garcia-Manero G, et al. 52. George B, Mathews V, Poonkuzhali B, et al. Treatment of children with newly diagnosed acute promyelocytic leukemia with arsenic trioxide: a single center experience. Leukemia. 2004; 18(10): 1587-1590. doi: 10.1038/sj.leu.2403480. 53. Zhang L, Zhao H, Zhu X, et al. Retrospective analysis of 65 Chinese children with acute promyelocytic leukemia: a single center experience. Pediatr Blood Cancer. 2008; 51(2): 210-215. doi: 10.1002/pbc.21510. 54. Fox E, Razzouk BI, Widemann BC, et al. Phase 1 trial and pharmacokinetic study of arsenic trioxide in children and adolescents with refractory or relapsed acute leukemia, including acute promyelocytic leukemia or lymphoma. Blood. 2008; 11(2): 566-573. doi: 10.1182/blood-2007-08-107839 55. Wang H, Hao L, Wang X, et al. Retrospective study of arsenic trioxide for childhood acute promyelocytic leukemia in China: a single-center experience. In J Hematol. 2010; 91(5): 820-825. doi: 10.1007/s12185-010-0575-z. 56. Zhang L, Zhu X, Zou Y, et al. Effect of arsenic trioxide on the treatment of children with newly diagnosed acute promyelocytic leukemia in China. In J Hematol. 2011; 93(2): 199-205. doi: 10.1007/s12185-011-0768-0 57. Cheng Y, Zhang L, Wu J, et al. Long-term prognosis of childhood acute promyelocytic leukemia with arsenic trioxide administration in induction and consolidation chemotherapy phases: a single-center experience. Eur J Haematol. 2013; 91(6): 483-489. doi: 10.1111/ejh.12194 58. Creutzig U, Dworzak MN, Bochennek K, et al. First experience of the AML-berlin-Frankfurt-Munster group in pediatric patients with standard-risk acute promyelocytic leukemia treated with arsenic trioxide and all-trans retinoic acid. Pediatr Blood Cancer. 2017; 64(8). doi: 10.1002/pbc.26461 59. Strocchio L, Gurnari C, Santoro N, et al. Arsenic trioxide and all-trans retinoic acid treatment for childhood acute promyelocytic leukemia. Br J Haematol. 2019; 185(2): 360-363. doi: 10.1111/bjh.15507 60. Schuurhuis GJ, Heuser M, Freeman S, et al. Minimal/measurable residual disease in AML: a consensus document from the European LeukemiaNet MRD Working Party. Blood. 2018; 131(12): 1275-1291. doi: 10.1182/blood-2017-09-801498 61. Zhu HH, Wu DP, Du X, et al. Oral arsenic plus retinoic acid versus intravenous arsenic plus retinoic acid for non-high-risk acute promyelocytic leukaemia: A non-inferiority, randomised phase 3 trial. Lancet Oncol. 2018; 19: 871-879. doi: 10.1016/S1470-2045(18)30295-X 62. Zhu HH, Wu DP, Jin J, et al. Oral tetra-arsenic tetra-sulfide formula versus intravenous arsenic trioxide as first-line treatment of acute promyelocytic leukemia: A multicenter randomized controlled trial. J. Clin. Oncol. 2013; 31: 4215-4221. doi: 10.1200/JCO.2013.48.8312 63. Wang L, Zhou GB, Liu P, et al. Dissection of mechanisms of Chinese medicinal formula Realgar-indigo naturalis as an effective treatment for promyelocytic leukemia. Proc Natl Acad Sci USA. 2008; 105: 4826-4831. doi: 10.1073/pnas.0712365105 64. The Cooperative Group of Phase II Clinical Trial of Compound Huangdai Tablet. Phase II clinical trial of compound Huangdai tablet in newly diagnosed acute promyelocytic leukemia. Clin J Hematol. 2006; 27: 801-804. 65. Zhu HH, Wu DP, Jin J, et al. Long-term survival of acute promyelocytic leukaemia patients treated with arsenic and retinoic acid. Br J Haematol. 2016; 174: 820-822. doi: 10.1111/bjh.13809. 66. Jiang H, Liang GW, Huang XJ, et al. Reduced medical costs and hospital days when using oral arsenic plus ATRA as the first-line treatment of acute promyelocytic leukemia. Leuk Res. 2015; 39: 1319-1324. doi: 10.1016/j.leukres.2015.09.007 67. Luo XQ, Ke ZY, Huang LB, et al. Improved outcome for Chinese children with acute promyelocytic leukemia: a comparison of two protocols. Pediatr Blood Cancer. 2009; 53: 325-328. doi: 10.1002/pbc.22042. 68. Wang J, Huang JB, Liu ZL, et al. Comparison of Curative Effect between Fu Fang Huang Dai Pian and Arsenic Trioxide in Treatment of 45 Patients with Acute Promyelocytic Leukaemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2017. 25: 1605–1610. doi: 10.7534/j.issn.1009-2137.2017.06.004 69. Au WY, Li CK, lee V, et al. Oral Arsenic Trioxide for Relapsed Acute Promyelocytic Leukemia in Pediatric Patients. Pediatr Blood Cancer. 2012; 58: 630-632. doi: 10.1002/pbc.23306 70. Yang MH, Wan WQ, Lou JS, et al. Multicenter randomized trial of arsenic trioxide and Realgar-Indigo naturalis formula in pediatric patients with acute promyelocytic leukemia: Interim results of the SCCLG-APL clinical study. Am J Hematol. 2018; 93: 1467-1473. doi: 10.1002/ajh.25271 71. Wang F, jia JS, Wang J, et al. The kinetics of white blood cell and the predictive factors of leukocytosis under oral or intravenous arsenic as the first line treatment for acute promyelocytic leukemia. Leuk Res. 2017; 61: 84-88. doi: 10.1016/j.leukres.2017.09.006 72. Tomita A, Kiyoi H, Naoe T. Mechanisms of action and resistance to all-trans retinoic acid (ATRA) and arsenic trioxide (As2O 3) in acute promyelocytic leukemia. Int J Hematol. 2013; 97: 717-725. doiI: 10.1007/s12185-013-1354-4 73. Takeshita A, Asou N, Atsuta Y, et al. Tamibarotene maintenance improved relapse-free survival of acute promyelocytic leukemia: a final result of prospective, randomized JALSG-APL 204 study. Leukemia. 2019; 33(2): 358-370. doi: 10.1038/s41375-018-0233-7. 74. Goldenson BH, Goodman AM, Ball ED. Gentuzumab ozogamicin for the treatment of acute myeloid leukemia in adults. Expert Opin Biol Ther. 2021; 21(7): 849-862. doi: 10.1080/14712598.2021.1825678 75. Walasec A. The new perspectives of targeted therapy in acute myeloid leukemia. Adv Clin Exp Med. 2019; 28(2): 271-276. doi: 10.17219/acem/81610. 76. Rasekh EO, Elsayed GM, Madney Y, et al. Prognostic significance of bcr1 and bcr-3 isoforms of PML-RARA and FLT3-ITD in patients with acute promyelocytic leukemia. Clin Lymphoma Myeloma Leuk. 2020; 20(3): 156-167. doi: 10.1016/j.clml.2019.08.006 77. Xu F, Yin CX, Wang CL, et al. Immunophenotypes and immune markers associated with acute promyelocytic leukemia prognosis. Dis Markers. 2014; 421906. doi: 10.1155/2014/421906 78. Silva WFD jr, Rosa LID, Marquez GL, et al. Real-life outcomes of acute promyelocytic leukemia in Brazil – early deaths are still a problem. Clin Lymphoma Myeloma Leuk. 2019; 19(2): e116-e122. doi: 10.1016/j.clml.2018.11.004 79. Sobas M, Czyz A, Montesinos P, et al. Outcome of a real-life population of patients with acute promyelocytic leukemia treated according to the PETHEMA guideline, The Polish Adult Leukemia Group (PALG) experience. Clin Lymphoma Myeloma Leuk. 2020; 20(2): 105-113. doi: 10.1016/j.clml.2019.09.616
Received: September 26, 2021; To cite this article : Testi AM, Musiu P, Piciocchi A, et al. Successes in the Evolution of Front-Line Therapy in Pediatric Acute Promyelocytic
Leukemia. British Journal of Cancer Research. 2021; 4:3. ©2021 Testi AM, et al.
Accepted: October 22, 2021;
Published: October 25, 2021.
