Protocol article / Open Access
DOI: 10.31488/bjcr.194
The Effect of Intravenous Iron in Treating Anemia in Ovarian Cancer Patients: A PhaseIii, Open-Label, Randomized Trial
Maryam Al-Hayki, MD*
1. Saskatchewan Cancer Agency, Regina, SK, Canada
*Corresponding author:Maryam Al-Hayki, MD, Principle Investigator Saskatchewan Cancer Agency, 4101 Dewdney Ave, Regina, SK, Canada S4T 7T1.
Abstract
Cancer related anemia is common occurring in more than 30% of patients at diagnosis, prior to initiation of antineoplastic therapy. Anemia is often considered a side effect of cancer therapy. It is present with most advanced cancer and associated with patient’s compromised performance status. Anemia is known to impact survival, disease progression, treatment efficacy, and the patient’s quality of life. Data on the predictive role of hemoglobin (Hgb) level on disease response to Cancer treatment are limited. A connection between iron metabolism and behavior of malignant ovarian tumor was found. Data on the effect of iron on malignant ovarian tumors are contradictory. There is limited evidence to guide clinical practice for IV iron supplementation in patients with cancer who are not receiving erythropoiesis-stimulating agents (ESA). Protocols for IV iron infusion are mainly based on experiences of patient blood management in non-oncologic patients, but no specific guidelines are available for oncologic patients and, in particular, patients with ovarian cancer. The plan in this study is to monitor participants’ Hgb and maintain it at 100 g/L in the treatment study group, by doing iron studies and giving intravenous iron infusion as needed. This study aims to study the role of intravenous (IV) iron infusion in the management of anemia in ovarian cancer patients. The safety and efficacy of IV iron infusion on anemia in ovarian cancer patients, and the effect on quality of life and overall survival will be assessed. This study will primarily operationalize the evaluation and treatment of anemia in cancer patients, with an emphasis on those who are receiving cancer treatment. Furthermore it will enable patients and physicians to individualize anemia treatment options based on patient condition.
Introduction
Incidence causes and effect of iron deficiency anemia in cancer patients
Anemia is prevalent in 30% to 90% of cancer patients [1]. This can be attributed to the disease process itself or its treatment, chemotherapy in particular [2].
Several studies have evaluated multiple factors leading to the development of anemia in cancer patients [3-5]. A large number of patients were found to be anemic prior to the initiation of any cancer treatment. The pathogenesis of anemia is complex and multifactorial, including perioperative bleeding, malnutrition, malabsorption, chronic inflammation, chronic debilitating co-morbidities and sequestration of hepcidin into macrophages, resulting in limited erythropoiesis in addition to chemotherapy-associated anemia. Seventy five percent of people on chemotherapy for various cancer types were found to be iron deficient, with 60 percent showing signs of absolute iron deficiency [1, 6].
Fatigue is the main symptom of anemia that can be associated with diminished physical functioning and decreased quality of life [7, 8]. Anemia can result in delay of treatment and, subsequently, might affect prognosis.
Effect of anemia on cancer and cancer treatment
Tumor oxygenation is dependent primarily on the availability of oxygen via the blood, and secondarily on the diffusional flux from the micro vessel to the oxygen-consuming cell. The availability process is a function of Hgb blood concentration, and the diffusional flux is controlled by the partial pressure of oxygen (pO2) gradient and on the diffusion distance between vessels and tumor cells [9-12].
However, tumor hypoxia has been extensively studied. Although hypoxia is lethal for many cells, a subpopulation of tumor cells are able to not only adapt to hypoxic conditions, but also become resistant to chemotherapy. The role of hypoxia in the phenomenon of therapy resistance has been acknowledged in the literature extensively.
Studies show that low cellular oxygen levels may promote more malignant phenotype and provoke tumor aggressiveness, resulting in malignant progression and decreased responsiveness to treatment [9-12].
Furthermore, hypoxia is able to promote tumour metastasis by inducing the expression of gene products involved in the metastatic cascade [13-15], and by stimulating neo angiogenesis [16]. It was reported that hypoxia may mediate selection for a more aggressive phenotype and enhance the clonal expansion of cells with diminished apoptotic potential [17]. Moreover, cellular hypoxia decreases tumor proliferation, thus compromising the effectiveness of most chemotherapeutic drugs that are primarily effective against rapidly dividing cells [18, 19].
Chemotherapy induced anemia
Chemotherapy induced anemia is quite common. Data on cancer patients receiving chemotherapy retrieved from pooled placebo arms of six randomized controlled trials (RCT) of erythropoietin, and from US community oncology electronic medical record (EMR) database, showed that 58% of patients in the RCTs and 46% EMR episodes had a Hgb decline from < 100 to < 90 g/L at week 9 of chemotherapy [20].
In a multicenter observational study of patients on chemotherapy for non-myeloid malignancies, almost half the patients (48%) had a Hgb level < 120 g/L [21].
A survey of Japanese patients receiving chemotherapy reported an average Hgb level prior to treatment of 95 g/L [22]. A similar study of chemotherapy-induced anemia in Denmark reported a median Hgb before transfusion of 90 g/L [23]. Furthermore, a European Cancer Anemia study reported a mean Hgb level in cancer patients before initiation of either iron supplementation, transfusion, or use of an Erythropoietin Stimulating Agents (ESA) of 97 g/L [24].
Correction of anemia in cancer patients
Recently, Clinical Practice Guidelines in Oncology Guidelines [25] for cancer and chemotherapy-induced anemia underwent substantial revision.
Erythropoietin
While a large body of data with respect to patients with chemotherapy-induced anemia has been generated from studies evaluating support with (ESAs), ESA use is widely restricted primarily due to cost and secondly due to significant adverse effects, including serious cardiovascular and thromboembolic events [26, 27].
The use of ESAs has decreased since 2005 because of data indicating inferior survival and worse cancer outcomes. The same was reported for ovarian cancer patients. Patients with ovarian cancer receiving ESAs had a mortality rate nearly 15% higher than patients not receiving ESAs [28].
Blood transfusion: Indications
In general, the uses and indications for transfusion therapy have changed over the last few years with a shift toward a more restrictive transfusion policy [29, 30].
Clinical practice guidelines from the American Association of Blood Banks [31], in regard to blood transfusion thresholds [29], advised that transfusion is not indicated until the Hgb level is 70-80 g/L with liberal thresholds (transfusion not indicated until the Hgb level is 90-100 g/L. Literature search for RCTs indicated that restrictive transfusion thresholds were not associated with higher rates of adverse clinical outcomes, including 30-day mortality, myocardial infarction, cerebrovascular accident, re-bleeding, pneumonia, or thromboembolism. However, co-morbidities or treatment that a patient is receiving should also be considered while deciding to prescribe a blood transfusion, especially for cancer patients receiving chemotherapy [1, 32-34].
Current guidelines recommend a restrictive transfusion policy for cancer-related anemia. Guidelines limit the use of transfusions to achieve a Hgb concentration of ≥ 70 g/L but acknowledge that transfusion may be reasonable when patients exhibit anemia symptoms or when they have comorbidities such as cardiac disease, chronic pulmonary disease, or cerebrovascular disease [25].
However, the benefit of a blood transfusion must be balanced with known risks that include the potential for transfusion-related reactions, such as transfusion-associated circulatory overload, transfusion-related acute lung injury, allergic reaction, febrile non-hemolytic transfusion reaction [30,35-37] and the potential for transmission of blood-borne pathogens [38].
Other limitations to consider include time, availability, and cost [39]. In addition, there is the inconvenience to both patients and healthcare professionals [27, 40, 41].
Pattern of blood transfusion
Transfusion requirements in cancer patients receiving myelosuppressive chemotherapy have not been prospectively studied [42, 43]. Data on transfusion practices show a wide variation in transfusion practices as it relates to cancer patients with anemia who are receiving chemotherapy [43-46].
Since the US Food and Drug Administration (FDA) advised restrictions on ESA use in 2007, a number of studies have shown a change in patterns of transfusion practice, indicating increase in transfusion frequency practice [33, 46 – 48].
A study indicated that clinical judgment and patient symptoms, not just Hgb value, were used in decisions to prescribe blood transfusions [49]. The study reported that the primary consideration for prescribing a blood transfusion was anemia symptoms in 72.1% of patients, with only 25.2% of patients prescribed a transfusion based exclusively on Hgb value. The mean Hgb level at which a decision to give blood transfusion was made ranged from 81 to 85 g/L.
Effect of blood transfusion on ovarian cancer
The effect of blood transfusions on survival in ovarian cancer patients is contradictory [50-52]. Perioperative packed red blood cell transfusion has been implicated as a negative prognostic marker in surgical oncology patients. Lindsay L, et al. [50] found that perioperative packed red blood cell transfusion in ovarian cancer patients, was not associated with an increased risk for recurrence or death. Whereas lower preoperative Hgb was associated with a higher risk for recurrence.
A retrospective study on 216 patients with advanced ovarian cancer reported a weak association between Hgb level and survival. Furthermore, a weak association between number of blood transfusion and poor survival has been identified. Where an average Hgb greater than 80 g/L during chemotherapy portends an improved overall survival, blood transfusion does not have any effect [51].
Another study done by Conor J et al. [52] reported poor survival is associated with the use of blood transfusion in ovarian cancer patients.
Iron and ovarian cancer
While some data suggest that high levels of systemic iron were found to be associated with increased risk for developing ovarian cancer [53- 55], other data suggest that iron might promote cancer cells apoptosis [56-59]. However, the clinical effect of iron supplement on cancer treatment outcome, tumor progression and survival has not been studied.
Some novel research explains that cancer cells are known to sequester iron, which can potentiate cancer progression through mechanisms that have not yet been completely elucidated. Iron uptake seemed to be connected to increased fatty acid production. Fatty acid production tends to be increased in cancer cells. Hence the connection to iron thought to be significant. Several links between fatty acids and iron metabolism have been identified. Fatty acids are essential building blocks for cell walls and for the cell signaling. Iron may be playing a critical role in increased fatty acid synthesis in cancer. Although the link between these processes and iron-related genes is not well understood, there is a theoretical concern that iron and iron-related genes impact and interact with fatty acid metabolic pathways and can promote tumorigenesis. It is not clear whether iron sequestration by cancer cells can potentiate cancer progression.
Contrary to the above, another group suggests that there might be a role for iron as a potent growth-suppressing agent in vitro for cell lines derived from ovarian cancer and a potential therapeutic drug to treat such tumors in vivo and, in particular, for platinum resistant tumors [60].
Current guidelines are inconclusive regarding intravenous iron for treatment of chemotherapy-induced anemia in ovarian cancer. There remains a lack of data regarding clinical factors that form the basis for making decisions on when to correct anemia with iron infusion in cancer patients and, in particular, ovarian cancer patients.
Oral Iron supplement
Oral iron supplement is less preferred than IV infusion in cancer patients and, in particular, ovarian cancer patients for the following reasons; poor adherence and tolerance in addition to a prolonged response compared to IV Iron infusion.
Intravenous iron preparations have improved over that seen historically with products such as high molecular weight iron dextran, which was associated with anaphylaxis and shock, including fatal events, and which have been largely removed from the market. The availability of IV iron formulations with improved toxicity profiles has lowered the threshold to consider switching from an oral to an IV preparation [61].
Iron infusion in cancer patients
A systematic review and meta-analysis of randomized controlled trials on the use of IV iron added to ESAs for the treatment of chemotherapy-induced anemia, reports a successful increase in hematopoietic response and reduction of blood transfusions, with no difference in mortality or adverse events [62]. Other data suggest use of IV iron without ESA in cancer patients, as it has been proven that IV iron alone can increase Hgb and stabilize Hgb levels at 110-120g/L [63].
IV iron has been tried with success in preventing anemia in the cervical cancer patients treated with concurrent chemo-radiotherapy [64]. Furthermore, intravenous iron has been used in anemic gynecologic cancer patients receiving anemic platinum-based chemotherapy, in which it resulted in reduced requirement of blood transfusion without serious adverse events [65].
Currently, there are different IV Iron product available. Unlike Dextran, all current IV Iron products are equally effective [66-70].
Response to iron infusion
In uncomplicated iron deficiency anemia, the patient might experience improved feeling of well-being within the first few days of treatment. The Hgb is expected to rise slowly, beginning 1-2 weeks after treatment, and will rise approximately 20 g/L over the 3rd week. Usually, the Hgb deficit would be halved by 4th week, and Hgb level would return to normal by 6th-8th week [71].
Response to iron infusion in ovarian cancer has not been studied before.
We expect a different response time in symptoms, Hgb, ferritin and TSAT might be observed in patients with advanced ovarian cancer, whether they are on active treatment or not.
In general, therapeutic options for anemia include iron replacement, administration of ESA and blood transfusion. The latter two should be kept at minimal use due to risks, cost and limited resources.
IV iron treatment results in a reduction in the need for blood transfusions and subsequently results in a reduction of transfusion-related adverse events. Increase in serum Hgb results in improvement of anemia-related symptoms and, subsequently, an improved quality of life. Furthermore, Hgb is found inversely dependent on modified Glasgow Prognostic Score [72].
There is limited evidence to guide clinical practice for IV iron supplementation in patients with cancer who are not receiving an ESA. Protocols for IV iron infusion are mainly based on experiences of Patient Blood Management (PBM) in non-oncologic patients, but no specific guidelines are available for oncologic patients and, in particular, patients with ovarian cancer.
Study Rationale
Ovarian cancer is the second most common gynecologic malignancy in Canada and the most common cause of gynecologic cancer death [73]. Most cases (80%) of ovarian cancer are diagnosed at advanced stage.
Most ovarian cancer patients, including both early and advanced stage, require extensive treatment including surgery and chemotherapy. Anemia occurs in more than 30% of patients with ovarian cancer at the time of diagnosis [3]. In patients with advanced disease in phase III trials performed by the Southwest Oncology Group, platinum-based first line chemotherapy was associated with a 33% red blood cell (RBC) transfusion rate [7]. The rate is probably similar for patients with recurrent ovarian cancer.
It has been reported in a retrospective study that 95% of ovarian cancer patients were anemic, and 26% of the patients had severe anemia [52].
Based on the above, we estimate that 95% of our patients will be anemic that requires correction. Cancer therapy and outcome might be affected adversely by anemia. Iron deficiency anemia (IDA) is the most common type of anemia. It is the major contributor to patients’ symptoms and delay in chemotherapy. Furthermore, it is the most potentially treatable cause.
Current guidelines are inconclusive regarding treatment of anemia in ovarian cancer. Most of the time, the treatment is individualized according to the patient’s symptoms.
Iron infusion has been successfully established in Saskatchewan for a few years. However, there are some questions that remain to be answered:
1. Is iron infusion feasible?
2. Is iron infusion safe for ovarian cancer patients?
3. Is Iron infusion during chemotherapy effective in correcting anemia and reducing need for blood transfusion?
4. In general, it takes 4 weeks to see a substantial increase in serum hemoglobin after iron infusion. Is that time altered in patients undergoing chemotherapy?
5. What is the effect of maintaining Hgb > 100 g/L on chemotherapy schedule?
6. What is the effect of iron infusion on quality of life (QOL) for ovarian cancer patients?
7. What is the effect of iron infusion on treatment outcome and overall survival?
Contribution of the study to patient care
Correction of anemia is vital in ovarian cancer patients to achieve the cancer treatment goal, alleviating symptoms of impaired exercise capacity and fatigue and improvement of quality of life. In addition, maintaining Hgb >100 g/L promotes adherence to cancer treatment and may positively influence therapeutic outcome.
This study aims to help in operationalizing and evaluating treatment of anemia in ovarian cancer patients. By assessing the effect of intravenous iron infusion on outcome, including quality of life and survival that would enable patients and physicians to individualize anemia treatment options. Iron infusion may reduce the requirements for blood transfusion.
The planned Study
This is a prospective, randomized controlled trial that will be conducted on eligible ovarian cancer patients registered at Saskatchewan Cancer Agency, Canada.
There will be a minimum of 200 patients enrolled over a 3-year time period.
Objectives
The objectives (Primary, Secondary) and their respective end points are listed below.
Primary objectives
1. Assessment of feasibility of iron infusion for ovarian cancer patients in Saskatchewan.
2. To assess overall safety and tolerability of iron infusion treatment in ovarian cancer patients.
3. To assess efficacy of iron infusion treatment in improving Hgb levels.
4. To assess response time to iron infusion in producing substantial increase in Hgb in ovarian cancer patients.
5. To assess the effect of maintaining Hgb > 100 g/L on chemotherapy schedule.
6. To observe the effect of iron infusion on requirement of blood transfusion in ovarian cancer patients.
7. To evaluate the efficacy of iron infusion on improving the quality of life (QOL) in ovarian cancer patients.
Secondary objectives
1. To evaluate the efficacy of iron infusion treatment on response to chemotherapy.
2. To assess the impact of iron infusion treatment on overall survival at 3 and 5 years.
Study Endpoints
Primary endpoints
1. Ensure the time needed to administer iron infusion, from the time when the treatment is requested by the treating physician to the time of IV iron administration on the participant.
2. Measure the safety of IV iron including type and frequency of adverse effects (AEs) and severity of adverse effect, discontinuation due to AEs and outcome of AE treatment. It will be compared to AEs (including frequency and severity of AEs, discontinuation due to AEs and outcome of AEs treatment) of blood transfusion and it will be compared to literature data on IV iron.
3. To assess the efficiency of IV iron, Hgb will be frequently assessed as per the study protocol. Hgb will be compared to baseline level, looking at substantial increase of Hgb of about 20 g/L increase within maximum 8 weeks (study estimated response time).
4. Time to response will be measured in every patient treated with IV iron from the time of treatment until substantial increase in Hgb from baseline 20 g/L. Hgb will be checked just prior to IV iron and then biweekly until week 8 or whenever Hgb rises at least by 20 g/L.
5. Timing of chemotherapy will be recorded and that will be compared to the standard treatment protocol. Any delay of chemotherapy schedule due to “anemia” will be flagged and recorded in all participants. These data will be compared between the two study groups.
6. Data of any blood transfusion will be collected, including the level of baseline pre-transfusion Hgb, indication of transfusion, number of transfusion units, AEs and frequency of blood transfusion episodes. Hgb level will be checked in transfused patients at 0, 2, 4, 6, and 8 weeks or until there is substantial increase of 20 g/L in Hgb compared to baseline. The collected data will allow comparison between the two study groups (A&B) and between patients who receive blood transfusion and patients who did not receive blood transfusion.
7. To measure any change from baseline QOL of both study groups and compare the difference of QOL in Group A (the group receiving IV infusion to correct anemia) compared to Group B. This will be done by comparing scores on the QOL questionnaire.
Secondary endpoints
1. Data on response to cancer treatment (all-chemo, radiation, PARP, etc.) will be collected in both groups. For chemotherapy patients, data will be collected before each treatment starting from the second treatment and then every three months after finishing chemotherapy. For other cancer treatments, response data will be collected every three months. Data on response will be compared between the two study groups (A & B). The disease-free survival (DFS) will be measured.
2. Survival rate of Group A compared to Group B at 3 years and 5 years.
Study Design
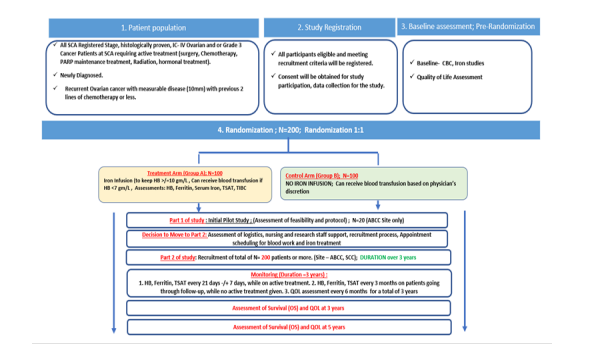
Figure 1:
Study Size and Time
A minimum of 200 patients will be enrolled over a 3-year time period.
Rationale for Sample Size
The Sample size has been calculated based on incidence of ovarian cancer patients in SK and based on likelihood of anemia in daily clinical practice. It is to be noted that it is not possible to divide Group A into static/fixed sub-groups based on treatment modality (surgery, chemo, PARP etc) as the nature of cancer is such that the course of planned treatment may change at any point in the disease trajectory due to the variable tumor/cancer kinetics and cellular environment. Therefore, Group A is considered as a single group with a non-homogenous mix rather than divide into further sub-groups.
Currently, there is no available data/study that reflects this study’s target population. As the population is non-homogenous. i.e mixed population cancer patients who may be under treatment modalities such as surgery, chemo, PARP treatment etc. For example, some patients who are surgical candidates may experience blood loss vs patients who are on chemo/PARP inhibitors and indication of blood transfusion indication in these sub-set of patients may be different.
Additionally, there is no current data or study that maintains Hb at 100g/L, however this study intents to maintain a Hb >100g/L. We expect the risk of blood transfusion to decrease by at least 25% (based on existing literature on preoperative IV iron treatment) in Group A. It is expected that there is enough sample size in Group A who will be receiving only iron treatment to be compared with Group B.
The sample size is calculated based on the achievement of Hgb ≥110 g/L (return to normal) after intravenous iron infusion. As intravenous iron infusion has a faster rise in Hgb level (rise of approximately 20 g/L over the 3rd week, halved by 4th week, and return to normal by 6th-8th week), it is assumed that 90% and 75% of the participants in the IV iron infusion (Iron Sucrose/Iron Gluconate/Iron Isomaltoside) and non IV Iron infusion (standard care) treatment groups, respectively, will have Hgb ≥110 g/L at the 6th-8th week of the clinical trial. At a 5% significance level and allowing the power to 80%, 200 participants are required for this study (100 in each treatment group) in order to consider the assumed difference between IV iron infusion and non IV iron infusion.
Method and Intervention
Health Organization (WHO) criteria for anemia in women are hemoglobin <120 g/L(WHO) and according to the National Cancer Institute (NCI)normal values for hemoglobin (Hgb) in women are 120 to 160 g/L. The National Comprehensive Cancer Network (NCCN) suggests evaluation of anemia if the hemoglobin is ≤110 g/L or if there is a decrease of ≥20 g/L below the individual's baseline (NCCN).
Diagnosis of anemia for this study protocol. Anemia will be classified as follow: mild anemia of Hgb 110-100 g/L, moderate anemia Hgb 100-80g/Land severe anemia Hgb < 70 g/L. Diagnosis of Iron deficiency anemia warranting Iron infusion in this study: Hgb < 100 g/L and /or low Ferritin (< 500 ng) and/or low TSAT (< 20%0.
The study treatment is divided into two groups (Arms):
Group A: Treatment study group
All patients will be treated with iron infusion for Hgb lower than 100 g/L. Blood transfusion may also be given based on physician’s discretion whenever indicated:
1. When Hgb level is < 70 g/L or in case of emergency and/or rapid blood loss.
2. Blood transfusion may be given to keep active treatment (chemotherapy, surgery, PARP inhibitors, hormonal, radiation) intervals as scheduled and not to exceed the maximum 4 weeks.
3. Based on current practice and NCCN guidelines, co-investigators/treating physicians are encouraged to avoid giving blood transfusion for Hgb >70g/L, provided the patient is stable and asymptomatic.
4. Blood transfusion can be combined with iron infusion.
5. Blood transfusion can be given if there is lack of response to iron infusion. Expected iron infusion response is expected at 8 weeks or less after treatment.
Group B: Control group
1. May receive blood transfusion when Hgb level is <70 g/L or in case of emergency and/or rapid blood loss.
2. Based on current practice and NCCN guidelines, co-investigators/treating physicians are encouraged to avoid giving blood transfusion for Hgb >70 g/L, provided the patient is stable and asymptomatic.
3. The decision to give blood transfusion for Hgb >70 g/L shall be based on the treating physician’s discretion:
a. symptomatic patient
b. to maintain active treatment schedule
c. to prepare the patient for surgery or an interventional procedure
Statistical Data Analysis
Since symptoms of malignancy and anemia are very much similar, we decided to use a QOL –short form to monitor improvement after iron infusion treatment[75-78].
Quality 0f Life –Short Form 12.
Data will be collected for both groups A & B at the time of recruitment and during follow up, including diagnosis and monitoring of anemia, response to IV iron, AEs, QOL, cancer treatment response and overall survival. Comparison will be conducted between the two groups.
For the main objectives of this study, data of the enrolled patients randomized in either the study treatment or control group will be compared. To this end, multiple regression analyses will be used to assess the effect of iron infusion (study treatment or control group membership), entered as an independent variable, on the patients’ quality of life (entered as the dependent variable). In addition, the effect of identified confounding variables will be assessed by entering these as independent variables into the equation. The same strategy will be employed in assessing the effect of iron infusion on other data from continuous variables.
The statistical analysis will be performed after data collection is completed, and the latest version of RStudio/SPSS/SAS/STATA will be used for statistical analyses.
Qualitative variables will be expressed as counts and percentages, and quantitative variables as mean standard deviation or median (interquartile range depending on the variable distribution). Continuous variables will be compared using the two-sided Student’s t-test or Mann-Whitney U test (where applicable), and the chi-squared test or Fisher’s exact test (where applicable) will be used to compare categorical variables.
The Kaplan-Meier method will be used for the graphical assessment of time-related events. The primary efficacy and safety endpoints will be analyzed by intention to treat. Participants who drop out during the trial will be set as censored. Cox proportional hazards regression using time-varying information on patient survival and adjusted for any potential confounding variables will be used to estimate hazard ratios and 95% confidence intervals. Differences will be considered statistically significant at P-values <0.05.
Stratified data will be considered during data analysis to help balance the risk of blood transfusion between Groups A and B should be considered and could be based on newly diagnosed vs recurrent cancer, history of blood transfusion, history of chemotherapy, moderate vs severe iron deficiency anemia at baseline etc.
Withdrawal Criteria
Participants can withdraw from the study at any time. In case of severe reaction to iron infusion, the participant can be switched to a different iron infusion drug (one of the study drugs) or be withdrawn from the study. The investigator can withdraw the patient from the study if there is a concern of patient’s safety or lack of treatment response (after 3 rounds/cycles of IV regimen).
Lack of response in this study is defined as lack to achieve a substantial increase in Hgb of at least 20 g/L from pre-IV iron treatment Hgb level.
References
1. Groopman JE, Itri LM. Chemotherapy-induced anemia in adults: incidence and treatment. 1999;91(19):1616–34.
2. Tas F, Eralp Y, Basaran M. Anaemia in oncology practice: relation to diseases and their therapies. 2004;11–26.
3. Macciò A. Hemoglobin levels correlate with interleukin-6 levels in patients with advanced untreated epithelial ovarian cancer: role of inflammation in cancer-related anemia. 2005;106(1):362–7.
4. Knight K, Wade S, Balducci L. Prevalence and outcomes of anemia in cancer: a systematic review of the literature. 2004;116(Suppl 7A):11S–26S.
5. Ludwig H, Van Belle S, Gascón P. Development, prediction, and treatment of anemia in patients (pts) with lymphoma/multiple myeloma (L/M): Findings of two European surveys (ECAS and BEPOS). 2004;104(11):3133–.
6. Schwartz RN. Anemia in patients with cancer: incidence, causes, impact, management, and use of treatment guidelines and protocols. 2007;64(3 Suppl 2):S5-13; quiz S28-30.
7. Harper P, Littlewood T.Anaemia of cancer: impact on patient fatigue and long-term outcome. 69(Suppl 2):2–7.
8. Hedenus M. Addition of intravenous iron to epoetin beta increases hemoglobin response and decreases epoetin dose requirement in anemic patients with lymphoproliferative malignancies: a randomized multicenter study. 2007;21(4):627–32.
9. Nieboer P. Fatigue and relating factors in high-risk breast cancer patients treated with adjuvant standard or high-dose chemotherapy: a longitudinal study. 2005;23(33).
10. Coleman CN, Mitchell JB, Camphausen K.Tumor hypoxia; chicken, egg, or a piece of the pharm. 20:pp. 610–615.
11. Feldmann HJ. Oxygenation of human tumors--implications for combined therapy. 2001;33(Suppl 1):S77–83
12. Höckel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. 93(4):266–76.
13. Wouters BG. Hypoxia as a target for combined modality treatments. 1990;38(2):240–57.
14. Graham CH. Hypoxia mediated stimulation of carcinoma cell invasiveness via up-regulation of urokinase receptor expression. 1999;80:617–23.
15. Xu L. Hypoxia-induced elevation in interleukin-8 expression by human ovarian carcinoma cells. 1999;59(22):pp. 5822–5829.
16. Canning MT. Oxygen-mediated regulation of gelatinase and tissue inhibitor of metallo-proteinases-1 expression by invasive cells. 367:88–94.
17. Semenza GL. Regulation of hypoxia-induced angiogenesis: a chaperone escorts VEGF to the dance. 108(1):39–40.
18. Graeber TG. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumors. 1996;379:88–91
19. Eisenhauer EA, Vermorken JB, van Glabbeke M. Predictors of response to subsequent chemotherapy in platinum pretreated ovarian cancer: a multivariate analysis of 704 patients [seecomments]. 1997;8(10):963–8.
20. Green SL, Giaccia AJ. Tumor hypoxia and the cell cycle: implications for malignant progression and response to therapy. 4(4):218–23.
21. Pirker R. Hemoglobin decline in cancer patients receiving chemotherapy without an erythropoiesis-stimulating agent. 2013;21(4):987–92.
22. Steegmann JL. Prevalence and management of anaemia in patients with non-myeloid cancer undergoing systemic therapy: a Spanish survey. 2013;15(6):477–83.
23. Tanaka A. Questionnaire-based survey on chemotherapy-induced anemia. 2014;19(3):411–20.
24. Yong M. Predictors and patterns of red blood cell transfusion use among newly diagnosed cancer patients with chemotherapy-associated anemia in Western Denmark (1998-2003). 2011;3:91–9.
25. Ludwig H, Van Belle S, Gascón P. Development, prediction, and treatment of anemia in patients (pts) with lymphoma/multiple myeloma (L/M): Findings of two European surveys (ECAS and BEPOS). 2004;104(11):3133–.
26. Available from: https://jnccn.org/view/journals/jnccn/10/5/article-p628.xml
27. Klarenbach S. Economic evaluation of erythropoiesis-stimulating agents for anemia related to cancer. 2010;116(13):3224–32.
28. Glaspy J. Update on safety of ESAs in cancer-induced anemia. 10(5):659–66.
29. Rocconi RP. Treatment of chemotherapy-induced anemia in ovarian cancer patients: Does the use of erythropoiesis-stimulating agents worsen survival. 22(5):786–91.
30. Carson JL. Clinical practice guidelines from the AABB: Red blood cell transfusion thresholds and storage. 316(19):2025.
31. Carson JL, Triulzi DJ, Ness PM. Indications for and adverse effects of red-cell transfusion. 2017;377(13):1261–72.
32. Xu L. Trends in anemia treatment among patients with five non-myeloid malignancies treated with chemotherapy in a large integrated health care delivery system in California, 2000–2013. 2013;.
33. Wilson J. A systematic review and economic evaluation of epoetin alpha, epoetin beta and darbepoetin alpha in anaemia associated with cancer, especially that attributable to cancer treatment. 2007;11(13):1–202, iii–v.
34. Hill SR.Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. 2002;(2):CD002042.
35. Khorana AA. Blood transfusions, thrombosis, and mortality in hospitalized patients with cancer. 2008;168(21):2377–81.
36. Schrijvers D. Management of anemia in cancer patients: transfusions. 2011;16(Suppl 3):12–8.
37. Blajchman MA, Vamvakas EC. The continuing risk of transfusion-transmitted infections. 2006;355(13):1303–5
38. Corey-Lisle PK. Transfusions and patient burden in chemotherapy-induced anaemia in France. 2014;6(4):146–53.
39. Berndt E. The impact of anaemia and its treatment on employee disability and medical costs. 2005;23(2):183–92.
40. Lyman GH. The economic burden of anemia in cancer patients receiving chemotherapy. 2005;8(2):149–56.
41. Watkins T, Surowiecka MK, McCullough J.Transfusion indications for patients with cancer. 22(1):38–46.
42. Laï-Tiong F. Management of anemia and iron deficiency in a cancer center in France. 2016;24(3):1091–6.
43. 8Aapro M. The background and methodology of the Anaemia Cancer Treatment (A.C.T.) study: a global retrospective study of practice patterns and outcomes in the management of anaemia in cancer patients and their congruence with evidence-based guidelines. 2008;16(2):193–200.
44. Ludwig H. Treatment patterns and outcomes in the management of anaemia in cancer patients in Europe: findings from the Anaemia Cancer Treatment (ACT) study. 2009;45(9):1603–15.
45. Ludwig H. A European patient record study on diagnosis and treatment of chemotherapy-induced anaemia. 2014;22(8):2197–206.
46. Hollingsworth K. Risk evaluation mitigation strategy: impact of application of the Food and Drug Adminstration’s strategy on use of erythropoiesis-stimulating agents and transfusion in patients with chemotherapy-induced anaemia. 2015;40(3):299–303.
47. Mouysset JL. Hemoglobin levels and quality of life in patients with symptomatic chemotherapy-induced anemia: the eAQUA study. 2016;8:1–0
48. Granfortuna J. Transfusion practice patterns in patients with anemia receiving myelosuppressive chemotherapy for nonmyeloid cancer: results from a prospective observational study. 26(6):2031–8.
49. Lindsay L. The impact of perioperative packed red blood cells transfusion on survival in epithelial ovarian cancer. 23(9):1612–9.
50. Altman AD. The effects of anemia and blood transfusion on patients with stage III-IV ovarian cancer. 23(9):1569–76.
51. Connor JP. Peri-operative allogeneic blood transfusion is associated with poor overall survival in advanced epithelial ovarian Cancer; potential impact of patient blood management on Cancer outcomes. 2018;151(2):294–8.
52. Konstorum A. A systems biology approach to understanding the pathophysiology of high-grade serous ovarian cancer: Focus on iron and fatty acid metabolism. 2018;22(7):502–13.
53. Basuli D. Iron addiction: a novel therapeutic target in ovarian cancer. 2017;36(29):4089–99.
54. Rockfield S. Iron overload and altered iron metabolism in ovarian cancer. 2017;398(9):995–1007.
55. Lange TS. Apoptotic and chemotherapeutic properties of iron (III)-salophene in an ovarian cancer animal model. 2009;3:17–26.
56. Singh RK. A novel indole ethyl isothiocyanate (7Me-IEITC) with anti-proliferative and pro-apoptotic effects on platinum-resistant human ovarian cancer cells. 2008;109(2):240–9.
57. Bauckman KA. Iron modulates cell survival in a Ras- and MAPK-dependent manner in ovarian cells. 2013;4(4):e592.
58. Bauckman K. Iron alters cell survival in a mitochondria-dependent pathway in ovarian cancer cells. 2015;466(2):401–13.
59. Zhang C, Zhang F. Iron homeostasis and tumorigenesis: molecular mechanisms and therapeutic opportunities. 6(2):88–100.
60. Gafter-Gvili A. Intravenous iron supplementation for the treatment of chemotherapy-induced anaemia - systematic review and meta-analysis of randomised controlled trials. 2013;52(1):18–29.
61. Steinmetz T. Clinical experience with ferric carboxymaltose as treatment of cancer-associated anemia. 2011;29(15_suppl):e19561.
62. Kim YT. Effect of intravenously administered iron sucrose on the prevention of anemia in the cervical cancer patients treated with concurrent chemoradiotherapy. 2007;105(1):199–204.
63. Dangsuwan P, Manchana T. Blood transfusion reduction with intravenous iron in gynecologic cancer patients receiving chemotherapy. 2010;116(3):522–5.
64. Auerbach M, Adamson JW. How we diagnose and treat iron deficiency anemia: Diagnose and treat iron deficiency anemia. 2016;91(1):31–8.
65. Auerbach M, Ballard H, Glaspy J. Clinical update: intravenous iron for anaemia. 2007;369(9572):1502–4.
66. Hetzel D. A Phase III, randomized, open-label trial of ferumoxytol compared with iron sucrose for the treatment of iron deficiency anemia in patients with a history of unsatisfactory oral iron therapy: Ferumoxytol Treatment in Iron Deficiency Anemia. 2014;89(6):646–50.
67. Macdougall IC.A randomized comparison of ferumoxytol and iron sucrose for treating iron deficiency anemia in patients with CKD. 9(4):705–12.
68. Kosch M. A randomized, controlled parallel-group trial on efficacy and safety of iron sucrose (Venofer) vs iron gluconate (Ferrlecit) in haemodialysis patients treated with rHuEpo. 2001;16(6):1239–44.
69. Kitsati N. Rapid elevation of transferrin saturation and serum hepcidin concentration in hemodialysis patients after intravenous iron infusion. 2014;100(3):80–3.
70. Macciò A. The role of inflammation, iron, and nutritional status in cancer-related anemia: results of a large, prospective, observational study. 2015;100(1):124–32.
71. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. 2020;70(1):7–30.
72. Heddens D. Factors associated with platinum-induced anemia in ovarian cancer (OVCA) patients (pts) in Southwest Oncology Group (S) studies. 1998;17:pp. 359. Available from: https://orthotoolkit.com/sf-12/
73. Ware Jr JE, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. 1996;34(3):220–33.
74. Gandek B. Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: results from the IQOLA Project. 1998;51(11):1171–8.
75. Jenkinson C. A shorter form health survey: can the SF-12 replicate results from the SF-36 in longitudinal studies?. 1997;19(2):179–86.
76. Ware JE, Keller SD, Kosinski M. SF-12: How to score the SF-12 physical and mental health summary scales. 1995.
Received: May 1, 2024;
Accepted: June 3, 2024;
Published: June 10, 2024.
To cite this article : Maryam Al-Hayki. The Effect of Intravenous Iron in Treating Anemia in Ovarian Cancer Patients: A Phase-Iii, Open-Label, Randomized Trial. British Journal of Cancer Research. 2024; 7(2): 687- 693. doi: 10.31488/bjcr.194.
© The Author(s) 2024. This is an open access article distributed under the terms of the Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0/).
