Case Report / Open Access
DOI: 10.31488/bjcr.182
The Effffect of Transcutaneous Electrical Nerve Stimulation on Chemotherapy Induced Neuropathic Pain and Mental Health in a Patient with Classic Hodgkin Lymphoma
Abdullah Alfouzan, BSc PT, DPT, CORS
Eastern Health Cluster, Dammam, Othman Ibn Affan, 32253 Saudi Arabia
*Corresponding author:Abdullah Alfouzan, BSc PT, DPT, CORS Department of physical therapy Eastern health cluster Dammam, Othman Ibn Affan, 32253 Saudi Arabia
Abstract
Background and Purpose: This case report examines the impact of Transcutaneous Electrical Nerve Stimulation (TENS) on chronic chemotherapy induced neuropathic pain and related depression in a male diagnosed with classic Hodgkin lymphoma (CHL). Case Description: a 36-year-old male complained of neuropathic pain in both feet following the first of six cycles of chemotherapy for CHL. Pain had persisted for four years afterwards, and the patient reported associated depression. Intervention: The patient received biphasic electrical stimulation using a portable TENS unit was delivered using electrodes placed bilaterally on the dorsum and soles of the feet for 30 minutes during each of two sessions each week for four weeks. The Patient Health Questionnaire-9 (PHQ-9) and the Short-form McGill Pain Questionnaire 2 (SF-MPQ-2) were used to evaluate pre-and post-outcomes. Outcomes: The results showed that following four weeks of TENS therapy, the patient’s neuropathic pain significantly decreased and his mental health significantly improved. Discussion: The findings support that TENS is effective in easing neuropathic pain in patients with CIPN and may improve related depression. Further investigation is warranted to study the long-term effect of TENS therapy on adults with CIPN to determine the most effective rehabilitation design. Studies should be conducted on the effect of TENS on patients with CIPN who are also suffering from depression symptoms.
Keywords: Electrical nerve stimulation, Hodgkin lymphoma, chemotherapy induced peripheral neuropathy, neuropathic pain
Background and Purpose
Chemotherapy-induced peripheral neuropathy (CIPN) is a frequent adverse side effect of various chemotherapy drugs [1]. Patients with CIPN complain of sensory symptoms such as numbness, tingling, hyperalgesia and allodynia, and some individuals may also experience poor motor coordination or autonomic dysfunction [2]. A systematic review and meta-analysis done by Seretny et al. has found that in the first month post chemotherapy almost 70% of patients experienced CIPN, and that 60% continued to have neuropathy 3 months afterwards [3]. The study also found that an estimated 30% percent of patients experienced ongoing neuropathic pain for 6 months or more [3]. This phenomenon of persisting CIPN is called “coasting” [4]. Risk factors for developing CIPN include a history of liver disease, renal disorder, peripheral vascular disease, diabetes, obesity, and chemotherapy type, with the latter three predicting the greater likelihood of CIPN [3,5-7].
Jones et al. has found CIPN is one of the adverse events that have a direct impact on the overall quality of life (QOL) of cancer survivors [8]. CIPN can impact the mental health of cancer survivors, particularly in terms of depression [9]. Hong et al. has found that patients with CIPN often experience psychological distress and a detrimental effect on the quality of sleep [10]. Moreover, depression can harm the ability of patients to manage their symptoms and participate in physical therapy (PT), leading to further declines in physical and mental well-being [11]. Depression may also lead to decreased adherence to chemotherapy or other treatments, potentially compromising the effectiveness of the treatments and the overall prognosis of cancer [12].
Transcutaneous electrical nerve stimulation (TENS) is an effective non-pharmacological treatment for pain due to its anti-hyperalgesic effect on the central nervous system through activation of delta and mu-opioid receptors in the brainstem [13]. TENS therapy has been shown to be effective in treatment of diabetic neuropathic pain [14,15]. Stepanovic et al. [16] reported that TENS was effective in preventing post-herpetic neuropathic neuralgia. Bilgili et al. found that TENS improved neuropathic pain in patients with complex regional pain syndrome [17]. Research by Gewandter et al. with patients with cancer primarily from a neuro-oncology clinic demonstrated that using home-based TENS to treat CIPN lowered numbness and tingling sensations significantly [18]. In another study of patients with CIPN who had unspecified types of cancer, Loprinzi et al. showed a 28% improvement in neuropathic pain after application of TENS [19]. A study by Vujadinovic et al. found TENS is effective in reducing neuropathic pain and enhancing QOL for patients with lung cancer [20]. Other studies have shown improvement in QOL after TENS application for conditions such as bladder hyperactivity and radiation induced neuropathy [21-22].
Classic Hodgkin Lymphoma (CHL), a type of cancer that originates in the lymphatic system, specifically affects the lymphatic cells, or lymphocytes, which help fight infection. CHL is most often found in the lymph nodes, but it can also affect other organs, such as the spleen, bone marrow, and liver [23]. According to the American Cancer Society (ACS) the median age of diagnosis is usually in the late 30s, and more males than females are affected. ACS has projected that by the end of 2023, there will be 8830 new diagnoses of CHL. It is also anticipated that the total number of annual deaths will be roughly 900 cases [24]. The five-year survival rate in the United States in all races and ethnicities between 2012 to 2018 was 85% [24]. The standard chemotherapy for CHL is doxorubicin, bleomycin, vinblastine and dacarbazine (ABVD) [25]; Geldof et al. found that vinblastine is neurotoxic [26]. Unfortunately, no studies have examined the prevalence of CIPN in CHL patients.
The purpose of this case report is to assess the short-term effect of post chemotherapy TENS intervention on neuropathic pain and depression in a patient with CHL with CIPN using the Short-form McGill Pain Questionnaire 2 (SF-MPQ-2) [27] and the Patient Health Questionnaire (PHQ-9) scale, respectively [28].
Case Description: Patient History and Systems Review
The patient was a 36-year-old male telecommunications customer service worker diagnosed with CHL stage lllB four years prior to the PT episode of care described in this case report. Per his medical record, magnetic resonance imaging revealed a mediastinal mass of 17x13cm and pleural and pericardial effusions. The pleural effusion was treated with intercostal tube insertion. He then received six cycles of the AVDB combination of chemotherapy drugs over a six-month period for a total of 12 treatments. The patient then underwent radiation therapy sessions to the chest for 2.5 months.
Following his first chemotherapy treatment, the patient reported experiencing numbness, tingling, burning and electric shock-like shooting pain in both his feet, which worsened while walking and improved slightly with self-massage. With the exception of burning, his symptoms persisted after the completion of chemotherapy and were not improved with analgesics including tramadol nor with gabapentin or pregabalin. After his fourth chemotherapy session, he was unable to actively dorsiflex or plantarflex his ankles. He reported that although his left dorsiflexors and plantar flexors eventually regained normal strength and his right plantar flexor strength improved without PT intervention, he remained unable to dorsiflex the right ankle. His physiatrist recommended that he use an ankle foot orthosis (AFO) to maintain a neutral position, but the patient stated he would only utilize it on occasion. Six months after the completion of chemotherapy, a nerve conduction velocity study showed that he had moderate to severe sensorimotor axonal polyneuropathy in his peroneal and tibial nerves, likely due to chemotherapy. Two years after the onset of his symptoms, he received PT for several months, which consisted of resistive exercises and calf muscle stretching. He continued to experience constant neuropathic pain, gait difficulties, and sleep disturbance. He also reported that he had lost interest in activities that he had previously enjoyed, such as reading books, walking in the shopping mall, playing sports, and going out with his friends. Two months prior to the PT episode of care described in this case report, his neurologist prescribed a second course of pregabalin which he took once a day. He reported that this alleviated his peripheral neuropathy and neuropathic pain for 3 to 4 hours, after which symptoms gradually returned, but with less tingling and numbness. He also reported more hours of sleep with an improved sleeping pattern.
The patient’s family history was unremarkable. His current medical history included psoriasis. He had several other comorbidities, including diabetes mellitus type 2, dyslipidemia, and hypertension. His medications included metformin, gliclazide, and linagliptin to manage diabetes, rosuvastatin for dyslipidemia, bisoprolol for hypertension and guselkumab for psoriasis. The patient’s additional comorbidities were obesity and fatty liver. He denied alcohol use. He had a 10 pack-year history of smoking tobacco but had quit one year prior.
The cardiopulmonary system assessment showed that his blood pressure was normal at 120/80 mm Hg. His heart rate of 90 was elevated, as was his respiratory rate of 22. There was no evidence of lower or upper extremity edema.
Integumentary system information was collected from the patient’s medical records. The patient had generalized scattered erythematous psoriatic plaques, with different sizes ranging from large, confluent irregular plaques on the lower extremities to smaller plaques on the extensor forearms, knees, face, scalp, elbows, and most of the upper extremities.
In the neuromuscular system the patient was noted to be experiencing issues with his gait, balance, and coordination. Despite this, the patient could perform transfers and transition between sitting and standing without assistance. The patient was able to ambulate without an assistive device independently. A right-sided steppage gait was observed. His cognitive and communication abilities appeared fully intact. He was alert, oriented to time, place, and person, and was fully cooperative and responsive to verbal commands. This was an essential factor in his PT as it allowed the patient to actively participate in therapy and understand the goals and instructions provided by the therapist.
From a musculoskeletal perspective, the patient was an obese individual with a height of 180 cm and weight of 145.75 kg, which calculates to a body mass index (BMI) of 45, which falls under the category of obese class III (BMI ≥40) [29]. His body was symmetrical and had a normal alignment. Leg lengths were equal. The patient’s lower extremity range of motion (ROM) was grossly within normal limits for all joints except for the right ankle and subtalar joints. Gross manual muscle testing (MMT) indicated normal lower extremity strength with the exception of the right leg and ankle musculature.
This patient’s goal for PT was to reduce neuropathic pain. Reducing the pain would help to improve the patient’s mood, sleep, and overall well-being.
Clinical Impression #I
The patient presented with the primary complaint of tingling and numbness in the feet, accompanied by electric shock-like shooting pain. The cause was most likely due to CIPN, although his diabetes mellitus and obesity might also have contributed, as both have been shown to play a role in reducing sensation in the lower limbs [30]. The patient first reported severe neuropathic symptoms in the feet after his first chemotherapy administration. Neuropathic pain is commonly seen in chemotherapy patients, particularly those receiving the drug vinblastine, which is a component of this patient’s AVDB chemotherapy regimen [26,30]. Additionally, the patient had a secondary complaint that his neuropathic pain caused a decline in his mood manifested by sleep disturbance and decreased pleasure in activities he used to enjoy.
The neurologist prescribed gabapentin to minimize the patient’s neuropathic pain, but after six months of taking the medication, there was no improvement in his symptoms. Exercise based PT was also unsuccessful. The patient had experienced only partial relief with Lyrica.
He was therefore considered a good candidate for TENS intervention which has been shown to relieve neuropathic pain, and positively affect related mental health issues, particularly depression [31].
The patient’s early nerve conduction velocity study which confirmed his neuropathy had not been repeated in the intervening 2 years. His examination plan therefore focused on lower extremity sensory and deep tendon reflex testing, testing of ROM and muscle strength, and assessment of balance and coordination. The plan also included a survey for neuropathic pain symptoms as well as other abnormal peripheral sensations, and a questionnaire for depression screening.
Examination
The examination process involved testing sensation to light touch, sharp/dull discrimination, and vibration in both feet. Light touch was assessed using a cotton swab on the dorsum and soles of the feet. The result showed that the patient could not feel light touch on either foot. Vibration testing was conducted by placing a tuning fork on various points on the feet, including the medial malleoli and the phalanges of the great toes. The patient was unable to detect vibration in any tested areas. Sharp and dull discrimination testing was conducted using a pin to apply a sharp stimulus on various points on the feet. The patient had normal feeling in all tested areas. Patellar and Achilles deep tendon reflexes testing showed that both deep tendon reflexes were absent bilaterally. The examination revealed that the patient had lost vibratory sensation and deep tendon reflexes, which was consistent with a persistent lower motor lesion [32-34].
The patient’s score on the Berg Balance Scale was 42, indicating moderate difficulty with balance and mobility [35]. He was unable to maintain his equilibrium on the Romberg test, which assessed the patient’s ability to maintain balance and coordination [36]. The positive result on the Romberg test indicated that the patient had a balance deficit and was at risk for falls.
The right ankle showed limited active ROM, with plantarflexion being limited to 0-15 degrees, dorsiflexion and eversion limited to 0 degrees, and inversion limited to 0-10 degrees. There were no restrictions in the passive mobility of the ankle.
MMT grades on the right were as follows: gastrocnemius 2/5, anterior tibialis 1/5, peroneus longus and brevis 0/5, and tibialis posterior -3/5. These findings of significant weakness further support that the patient’s peripheral nerve injury previously demonstrated by nerve conduction velocity testing was unresolved.
Neuropathic pain symptoms, as well as other abnormal peripheral sensations, were measured by the SF-MPQ-2, a self-reported questionnaire that measures the quality and intensity of the patient’s pain [27]. The SF-MPQ-2 has 22 items of pain descriptors divided into 4 sub-scales. An overall pain score is produced by averaging the patient’s ratings across all questions, whereas subscale pain scores are obtained by averaging ratings across questions representing the corresponding scales [37]. The severity of each descriptor is rated from 0 to 10. A user license agreement for the SF-MPQ-2 was obtained from the Mapi Research Trust [38] and an Arabic version of the questionnaire (Table 1) [38] was administered to the patient prior to the first TENS treatment. Out of possible scores of 10, the patient’s overall score was 3.90 and his neuropathic pain subscale score was 3.66. Mental health status, particularly depression, was also assessed using an Arabic version of the PHQ-9 survey [39] (Table 2). The depression severity is determined by scoring each of the 9 items one of the following response options: 0 (never), 1 (some days), 2 (over half the days), and 3 (almost every day) [40]. The patient’s score was 7 out of a possible 27, which indicated mild depression [41]. The scores on the two instruments suggested that the patient was experiencing neuropathic pain with related depression.
Table 1.Short-form McGill Pain Questionnaire (SF-MPQ-2) Arabic Version

Table 2.Patient Health Questionnaire-9 (PHQ-9)
The examination process helped to establish a baseline for the patient’s neuropathic pain and mental health status before TENS therapy, which allowed for more accurate assessment of the effectiveness of TENS in reducing neuropathic pain and improving related mental health status.
Clinical Impression #ll
Given his diagnosis of CHL and the appearance of neuropathic pain during chemotherapy administration, the patient in question was an appropriate candidate for TENS therapy for one month.
Neuropathic pain is typically difficult to treat with traditional therapy [42]. TENS is a pain relieving technique that is non-invasive. It has been shown that this type of treatment effectively reduces pain in people with various conditions, including neuropathic pain brought on by chemotherapy [18-20]. The plan was to evaluate the patient immediately before the first treatment session and again at the conclusion of the treatments, using the SF-MPQ-2 and PHQ-9 scales to evaluate the efficacy of TENS therapy on his pain and mental health. TENS therapy has shown promising results in reducing neuropathic pain in patients with cancer as well as psychological distress in other populations with pain [43].
A successful intervention would be indicated if the patient had an improvement in mental health as measured by the PHQ-9 and a clinically significant decrease in pain intensity/severity as measured by the SF-MPQ-2.
Intervention
For this case report, all the possible risks and benefits of the treatment were reviewed with the patient, and his consent to participate was obtained. The TENS unit used was a 2-channel symmetrical biphasic waveform stimulator with customizable programming (TensMed-S82, Enraf-Nonius b.v. Netherlands). Conventional TENS parameters include high-frequency (50-100 Hz), low-intensity, and small pulse width (50-200s) [44]. Based on Vujadinovic et al. protocol for treating neuropathic pain, the parameters chosen were a frequency of 80 pulses per second and a pulse width of 200 μs delivered for 30 minutes at a maximum intensity of 60 mA [20]. The patient was instructed to practice deep breathing exercises during the TENS sessions as part of his chronic pain management. Alcohol wipes were used on the dorsum and soles of the feet to decrease the skin impedance before applying two adhesive electrodes bilaterally on the dorsum and soles of each foot (Figure 1). The intensity was increased until the patient reported a mild tingling without muscle twitch. The intensity was then gradually increased up to 60 mA during each session as long as the patient reported a tolerable sensation without discomfort or motor response. The frequency of the intervention was two sessions of 30 minutes every week for four weeks.
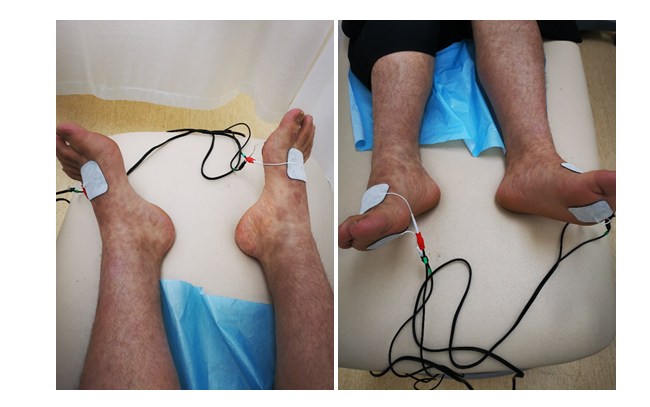
Figure 1.Electrode placement sites
During TENS interventions the patient received education on exercise, weight loss and nutrition to better manage his hypercholesteremia and diabetes mellitus. He was also instructed in the importance of using his AFO and stretching his calf muscles. Additional therapy sessions for therapeutic exercise were planned after the conclusion of the 4 weeks of TENS. The patient was evaluated again at the conclusion of the 4 weeks using the SF-MPQ-2 and the PHQ-9 scale.
Outcome
The SF-MPQ-2 has been found to have good test-retest reliability with a correlation of 0.83 and strong internal consistency with a Cronbach’s alpha of 0.96 [45]. The minimal clinically important difference (MCID) for the SF-MPQ-2 is 1-2.3 [46]. The PHQ-9 has been shown to be a valid and reliable predictor of severity of depression, with an MCID of 2.0-4.8 [41,47].
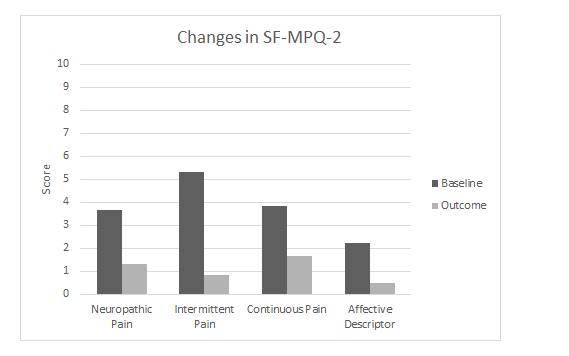
Figure 2.Changes in the means of the 4 sub-scales of the Short-form McGill Pain Questionnaire 2 (SF-MPQ-2) from preintervention (baseline) to postintervention at the conclusion of 4 weeks of TENS therapy (outcome).
The patient’s pre-intervention and post TENS intervention scores on the SF-MPQ-2 and PHQ-9 are shown in Figure 2. The post-intervention SF-MPQ-2 score reduction for all 22 items was 2.7, a clinically significant improvement. The score for the 6 items that comprise the neuropathic pain subscale decreased by 2.64 points from the baseline, but the MCID for this subscale has not been previously reported. The post-intervention PHQ-9 score decreased 5 points from the baseline, a substantially significant improvement in depression symptoms after the TENS intervention.
Discussion
The outcomes of this case report suggest that TENS therapy can effectively manage neuropathic pain and improve associated depression in CHL patients experiencing neuropathy from chemotherapy. A strength of this study is that the patient received no other PT interventions other than patient education, making it more likely that the outcomes are attributable to TENS. Although a study by Vondracek et al. has shown a significant improvement in neuropathic pain with the medication Lyrica that this study’s patient was taking during the TENS intervention, the patient’s improvement was more likely to have been due to TENS as his CIPN had not changed during a previous course of Lyrica [48]. While no previous studies have examined the effect of TENS on depression symptoms in the CIPN population, this case report’s outcomes support Coyne’s finding that TENS improved QOL in patients with radiation induced neuropathy [49].
This case report supports the pulse width, pulse frequency, and minutes of treatment used by Gewandter et al. and Vujadinovic et al. to reduce CIPN [18,20]. However, Loprinzi et al. effectively utilized a lower frequency (43 to 52 Hz) with a lower intensity (3.50 to 5.50 mA) and twice the duration of treatment per session (60 minutes), indicating a need for further research to determine the most effective parameters [19].
There are many benefits to using TENS to treat neuropathic pain. It is non-invasive and simple to administer at home or in a medical setting. It is also a low-cost intervention with few adverse effects, which makes it a desirable choice for people with limited resources. In addition, it can be employed with few resources and time constraints because it is quick and simple to implement, and it is an excellent option for those unable to receive more intensive treatments.
A barrier to using TENS to treat CIPN may be some PTs' caution regarding the safety of applying electrical stimulation to patients with cancer. A systematic review published by Siemens et al concluded that application of TENS in hospitalized palliative care patients with advanced cancer pain is a safe intervention [50]. Another systematic review has shown TENS can be safely used in an effort to reduce pain and CIPN in patients with cancer [51]. The side effects of TENS are generally considered mild, and include skin irritation at the site of the electrodes [50]. Some patients may also experience discomfort during therapy [52]. However, these side effects are typically temporary and resolve independently once the intervention is terminated [19].
There were several limitations of this case report. According to Greenlee et al., people who are obese while undergoing chemotherapy frequently exhibit symptoms of CIPN [6]. Another study by Hershman et al. [5] has demonstrated that diabetic patients who receive chemotherapy are twice as likely to get CIPN. Diabetes and obesity are also risk factors for developing neuropathy unrelated to chemotherapy. The patient in this case report had both comorbidities, and while it is most likely that his neuropathy was caused by his chemotherapy, it is not certain. Another limitation is that he has not had recent nerve conduction velocity testing to evaluate any changes in the past two years. Due to transportation difficulties, the patient was unable to receive therapy 5 days a week, as was done in the study by Vujadinovic et al. [20]. Had he been able to receive more treatment, he may have made larger gains.
Several areas require further research to understand TENS’ full potential in the targeted patient population. Firstly, TENS’ efficacy as an adjunct during or immediately following chemotherapy treatment must be investigated. Additionally, research should be conducted to determine if TENS is effective in treating other side effects of chemotherapy, such as fatigue and nausea. The long-term effectiveness of TENS in reducing CIPN pain levels should also be studied. The optimal electrode location for CIPN should be determined. Finally, the most effective TENS parameters, such as frequency, pulse width and duration, needed to control CIPN most effectively should be investigated.
Overall, the results of this study provide a solid foundation for future research in this area and highlight the potential of TENS as a non-invasive and low-cost therapy for CIPN and related mental health issues in patients with CHL as well as other cancers.
References
1. Zajączkowska R, Kocot-Kępska M, Leppert W, Wrzosek A, Mika J, Wordliczek J. Mechanisms of Chemotherapy-Induced Peripheral Neuropathy. International Journal of Molecular Sciences. 2019;20(6):1451. doi:10.3390/ijms20061451
2. Miltenburg NC, Boogerd W. Chemotherapy-induced neuropathy: A comprehensive survey. Cancer Treat Rev. 2014;40(7):872-882. doi:10.1016/j.ctrv.2014.04.004
3. Seretny M, Currie GL, Sena ES, et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain. 2014;155(12):2461-2470. doi:10.1016/j.pain.2014.09.020
4. Grisold W, Cavaletti G, Windebank AJ. Peripheral neuropathies from chemotherapeutics and targeted agents: diagnosis, treatment, and prevention. Neuro Oncol. 2012;14 Suppl 4(Suppl 4):iv45-iv54. doi:10.1093/neuonc/nos203
5. Hershman DL, Till C, Wright JD, et al. Comorbidities and Risk of Chemotherapy-Induced Peripheral Neuropathy Among Participants 65 Years or Older in Southwest Oncology Group Clinical Trials. J Clin Oncol. 2016;34(25):3014-3022. doi:10.1200/JCO.2015.66.2346
6. Greenlee H, Hershman DL, Shi Z, et al. BMI, Lifestyle Factors and Taxane-Induced Neuropathy in Breast Cancer Patients: The Pathways Study. J Natl Cancer Inst. 2016;109(2):djw206. Published 2016 Oct 28. doi:10.1093/jnci/djw206
7.Quasthoff S, Hartung HP. Chemotherapy-Induced Peripheral Neuropathy. J Neurol. 2002;249(1):9-17. doi:10.1007/pl00007853
8.Jones D, Zhao F, Brell J, et al. Neuropathic symptoms, quality of life, and clinician perception of patient care in medical oncology outpatients with colorectal, breast, lung, and prostate cancer. J Cancer Surviv. 2015;9(1):1-10. doi:10.1007/s11764-014-0379-x
9. Kim KY, Lee SH, Oh PJ. Chemotherapy-induced Peripheral Neuropathy and Depression in Cancer Patients. Asian Oncol Nurs. 2015;15(3):149-155. Doi:10.5388/aon.2015.15.3.149
10. Hong JS, Tian J, Wu LH. The Influence of Chemotherapy-Induced Neurotoxicity on Psychological Distress and Sleep Disturbance in Cancer Patients. Current Oncology. 2014; 21(4):174-180.
11. Jack K, McLean SM, Moffett JK, et al. Barriers to treatment adherence in physiotherapy outpatient clinics: a systematic review. Man Ther. 2010;15(3):220-228. doi:10.1016/j.math.2009.12.004
12. De Souza BF, de Moraes JA, Inocenti A, et al. Women with breast cancer taking chemotherapy: depression symptoms and treatment adherence. Rev Lat Am Enfermagem. 2014;22(5):866-873. doi:10.1590/0104-1169.3564.2491
13. Kalra A, Urban MO, Sluka KA. Blockade of opioid receptors in rostral ventral medulla prevents antihyperalgesia produced by transcutaneous electrical nerve stimulation (TENS). J Pharmacol Exp Ther. 2001;298(1):257-263.
14. Al-Zamil M, Minenko IA, Kulikova NG, et al. Clinical Experience of High Frequency and Low Frequency TENS in Treatment of Diabetic Neuropathic Pain in Russia. Healthcare (Basel). 2022;10(2):250. Published 2022 Jan 28. doi:10.3390/healthcare10020250
15. Jin DM, Xu Y, Geng DF, et al. Effect of transcutaneous electrical nerve stimulation on symptomatic diabetic peripheral neuropathy: a meta-analysis of randomized controlled trials. Diabetes Res Clin Pract. 2010;89(1):10-15. doi:10.1016/j.diabres.2010.03.021
16. Stepanović A, Kolšek M, Kersnik J, et al. Prevention of post-herpetic neuralgia using transcutaneous electrical nerve stimulation. Wien Klin Wochenschr. 2015;127(9-10):369-374. doi:10.1007/s00508-014-0669-3
17. Bilgili A, Çakır T, Doğan ŞK, et al. The effectiveness of transcutaneous electrical nerve stimulation in the management of patients with complex regional pain syndrome: A randomized, double-blinded, placebo-controlled prospective study. J Back Musculoskelet Rehabil. 2016;29(4):661-671. doi:10.3233/BMR-160667
18. Gewandter JS, Chaudari J, Ibegbu C, et al. Wireless transcutaneous electrical nerve stimulation device for chemotherapy-induced peripheral neuropathy: an open-label feasibility study. Supportive Care in Cancer. 2018;27(5):1765-1774. doi:10.1007/s00520-018-4424-6
19. Loprinzi C, Le-Rademacher JG, Majithia N, et al. Scrambler therapy for chemotherapy neuropathy: a randomized phase II pilot trial. Support Care Cancer. 2020;28(3):1183-1197. doi:10.1007/s00520-019-04881-3
20. Vujadinovic ST, Ilic N, Selakovic I, et al. TENS Improves Cisplatin-Induced Neuropathy in Lung Cancer Patients. Medicina (Kaunas). 2022;58(10):1405. Published 2022 Oct 6. doi:10.3390/medicina58101405
21. Götzl R, Sterzinger S, Semrau S, et al. Patient's quality of life after surgery and radiotherapy for extremity soft tissue sarcoma - a retrospective single-center study over ten years. Health Qual Life Outcomes. 2019;17(1):170. Published 2019 Nov 8. doi:10.1186/s12955-019-1236-4
22. Leão S Santos H, Caldwell P, Hussong J, et al. Quality of life and psychological aspects in children with overactive bladder treated with parasacral transcutaneous electrical nerve stimulation - A prospective multicenter study. J Pediatr Urol. 2022;18(6):739.e1-739.e6. doi:10.1016/j.jpurol.2022.10.011
23. Gobbi PG, Ferreri AJM, Ponzoni M, et al. Hodgkin lymphoma. Critical Reviews in Oncology/Hematology. 2013;85(2):216-237. doi:10.1016/j.critrevonc.2012.07.002
24. Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17-48. doi:10.3322/caac.21763
25. Hanel W, Herrera AF, Epperla N. Management of classical Hodgkin lymphoma: a look at up to date evidence and current treatment approaches. Exp Hematol Oncol. 2022;11(1):108. Published 2022 Dec 27. doi:10.1186/s40164-022-00360-4
26. AA Geldof, A Minneboo, JJ Heimans. Vinca-alkaloid neurotoxicity measured using an in vitro model. Journal of neuro-oncology vol. 37,2 (1998): 109-13. doi:10.1023/a:1005848623771
27. Dworkin RH, Turk DC, Revicki DA, et al. Development and initial validation of an expanded and revised version of the Short-form McGill Pain Questionnaire (SF-MPQ-2). Pain. 2009;144(1-2):35-42. doi:10.1016/j.pain.2009.02.007
28. Negeri ZF, Levis B, Sun Y, et al. Accuracy of the Patient Health Questionnaire-9 for screening to detect major depression: updated systematic review and individual participant data meta-analysis. BMJ. 2021;375:n2183. doi:10.1136/bmj.n2183
29. Kok P, Seidell JC, Meinders AE. The value and limitations of the body mass index (BMI) in the assessment of the health risks of overweight and obesity. Nederlands tijdschrift voor geneeskunde. 2017 Nov 1;148(48):2379-82.
30. Eskut N, Koskderelioglu A. Neurotoxic agents and peripheral neuropathy. Neurotoxicity - New Advances. 2022. doi:10.5772/intechopen.101103
31. Johnson M. Transcutaneous Electrical Nerve Stimulation: Mechanisms, Clinical Application, and Evidence. Reviews in Pain. 2007;1(1):7-11. doi:10.1177/204946370700100103
32. Klingner CM, Witte OW. Somatosensory deficits. Handbook of Clinical Neurology. Published online 2018:185-206. doi:10.1016/b978-0-444-63622-5.00009-7
33. Lin-Wei O, Xian LLS, Shen VTW, et al. Deep Tendon Reflex: The Tools and Techniques. What Surgical Neurology Residents Should Know. Malays J Med Sci. 2021;28(2):48-62. doi:10.21315/mjms2021.28.2.5
34. Kersten P. Principles of physiotherapy assessment and outcome measures. Physical Management in Neurological Rehabilitation. Published online 2004:29-46. doi:10.1016/b978-072343285-2.50007-3
35. Sahin F, Yilmaz F, Ozmaden A, et al. Reliability and validity of the Turkish version of the Berg Balance Scale. Journal of geriatric physical therapy. 2018 Jan 1;31(1):32-7.
36. Forbes J, Munakomi S, Cronovich H. Romberg Test. In: StatPearls. Treasure Island (FL): StatPearls Publishing; November 2, 2022.
37. Lovejoy TI, Turk DC, Morasco BJ. Evaluation of the psychometric properties of the revised short-form McGill Pain Questionnaire. J Pain. 2012;13(12):1250-1257. doi:10.1016/j.jpain.2012.09.011
38. Melzack R, Immpact Group. Short-form McGill Pain Questionnaire (SF-MPQ-2).mapi-trust.org. published April 7, 2009.
39. Kroenke K. Welcome to the patient health questionnaire (PHQ-9) screeners. PHQ screeners.com. Published 2002.
40. Kroenke K, Spitzer RL, Williams JB, et al. The Patient Health Questionnaire Somatic, Anxiety, and Depressive Symptom Scales: a systematic review. Gen Hosp Psychiatry. 2010;32(4):345-359. doi:10.1016/j.genhosppsych.2010.03.006
41. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613. doi:10.1046/j.1525-1497.2001.016009606.x
42. Schug SA, Stannard KJ. Treatment of Neuropathic Pain - Mechanisms of Vascular Disease - NCBI Bookshelf. Published January 1, 2011.
43.Altay F, Durmus D, Canturk F. Effects of TENS on Pain, Disabiliy, Quality of Life and Depression in Patients with Knee Osteoarthritis. Turkish Journal of Rheumatology. 2010;25(3):116-121. doi:10.5152/tjr.2010.14
44. Charlton JE. Core Curriculum for Professional Education in Pain.; 2005. doi:10.1604/9780931092657
45. Jumbo SU, MacDermid JC, Kalu ME, et al. Measurement Properties of the Brief Pain Inventory-Short Form (BPI-SF) and Revised Short McGill Pain Questionnaire Version-2 (SF-MPQ-2) in Pain-related Musculoskeletal Conditions: A Systematic Review. Clin J Pain. 2021;37(6):454-474. doi:10.1097/AJP.0000000000000933
46. Sabourin S, Tram J, Sheldon BL, et al. Defining minimal clinically important differences in pain and disability outcomes of patients with chronic pain treated with spinal cord stimulation [published online ahead of print, 2021 Jun 4]. J Neurosurg Spine. 2021;1-8. doi:10.3171/2020.11.SPINE201431
47. Lynch CP, Cha EDK, Jenkins NW, et al. The Minimum Clinically Important Difference for Patient Health Questionnaire-9 in Minimally Invasive Transforaminal Interbody Fusion. Spine (Phila Pa 1976). 2021;46(9):603-609. doi:10.1097/BRS.0000000000003853
48. Vondracek P, Oslejskova H, Kepak T, et al. Efficacy of pregabalin in neuropathic pain in pediatric oncological patients. Eur J Paediatr Neurol. 2009;13(4):332-336. doi: 10.1016/j.ejpn.2008.06.011
49.Coyne PJ, Wan W, Dodson P, et al. A trial of Scrambler therapy in the treatment of cancer pain syndromes and chronic chemotherapy-induced peripheral neuropathy. J Pain Palliat Care Pharmacother. 2013;27(4):359-364. doi:10.3109/15360288.2013.847519
50. Siemens W, Boehlke C, Bennett MI, et al. Transcutaneous electrical nerve stimulation for advanced cancer pain inpatients in specialist palliative care-a blinded, randomized, sham-controlled pilot cross-over trial. Support Care Cancer. 2020;28(11):5323-5333. doi:10.1007/s00520-020-05370-8
51. Püsküllüoğlu M, Tomaszewski KA, Grela-Wojewoda A, et al. Effects of Transcutaneous Electrical Nerve Stimulation on Pain and Chemotherapy-Induced Peripheral Neuropathy in Cancer Patients: A Systematic Review. Medicina (Kaunas). 2022;58(2):284. doi:10.3390/medicina58020284
52. Boggio PS, Amancio EJ, Correa CF, et al. Transcranial DC stimulation coupled with TENS for the treatment of chronic pain: a preliminary study. The Clinical journal of pain. 2019;25(8):691-5.
Received: May 15, 2023;
Accepted: June 05, 2023;
Published: June 12, 2023.
To cite this article : Alfouzan A. The Effect of Transcutaneous Electrical Nerve Stimulation on Chemotherapy Induced Neuropathic Pain and Mental Health in a Patient with Classic Hodgkin Lymphoma. British Journal of Cancer Research. 2023; 6(1): 604- 612. doi: 10.31488/bjcr.182.
©2023 Alfouzan A.
