Research article / Open Access
DOI: 10.31488/bjcr.180
Treatment of Vaginal Atrophy Using Fractional Microablative CO2 Laser in Post-Menopausal Women with Breast Cancer on Aromatase Inhibitors: A Pilot Study
Rebecca Tay*1,2, Kate Stewart3, Genevieve Read1,4,5, W. Catarina Ang1,4, Mohammad Asghari-Jafarabadi1,6, and Yoland Antill1,7
1. Cabrini Research, Cabrini Health, Melbourne, VIC, 3144, Australia
2. Royal Hobart Hospital, Hobart, TAS, 7000
3. Department of Anatomical Pathology, Austin Health, Heidelberg, VIC, 3084
4. Royal Women’s Hospital, 20 Flemington Rd, Parkville 3052
5. Latrobe Regional Hospital, Traralgon, 3844
6. School of Public Health and Preventative Medicine, Faculty of Medicine, Nursing and Health Sciences, Monash University, Melbourne, VIC, 3800, Australia
7. Faculty of Medicine, Nursing and Health Science, Monash University, Wellington Rd & Blackburn Rd, Clayton VIC 3800
*Corresponding author:Rebecca Tay, Cabrini Research, Cabrini Health, Melbourne, VIC, 3144, Australia
Abstract
Aim: Fractional CO2 intra-vaginal laser is a non-hormone-based therapy designed to treat vaginal atrophy (VA). The feasibility of CO2 laser in post-menopausal women with breast cancer (BC) on aromatase inhibitors (AI) was evaluated.Method Stage I-III post-menopausal BC patients on an AI with symptomatic VA received 3 CO2 laser treatments scheduled 4 weeks apart. Clinical assessment, vaginal cytology, biopsy and patient questionnaires were undertaken at baseline and 12-week post treatment. The primary endpoint was improvement in the Urogenital Atrophy Questionnaire (UAQ) and the Vaginal Health Index Score (VHIS). Secondary end points included improvement in sexual function on Female Sexual Function Index (FSFI) and change in vaginal epithelial cytology and histology. Results Between May 2017 and July 2020, 33 patients were enrolled. 27 patients completed all 3 pre-planned treatments and post-treatment assessment (T1 n=30; T2 n=30; T3 n=28); post-Rx n=27). Patient-reported vaginal dryness (100 vs 48%, p=<0.001), irritation/itch (56 vs 19%, p=0.008), pain (63 vs 11%, p=0.001) and dyspareunia (89 vs 78%, p=0.371) reduced at 12-weeks post treatment. VHIS increased by a mean 4.1-points at final assessment (p=<0.001). Patient reported vaginal dryness improved on UAQ (p=<0.001). No clinically meaningful improvement in sexual function domains were detected on UAQ and FSFI questionnaires. Conclusions CO2 laser is a feasible non-hormonal option for post-menopausal BC patients on AI therapy resulting in improvements in patient-reported vaginal dryness and clinician-assessed VA. This intervention is less effective for treating sexual function symptoms that may be associated with VA.
Keywords :Vaginal atrophy, genitourinary syndrome, laser,menopause, breast cancer
Introduction
Genitourinary Syndrome of Menopause (GSM) is caused by the reduction of circulating oestrogen resulting in vaginal atrophy, epithelial inflammation and changes to vaginal pH and flora [1,2]. Symptoms of GSM include vaginal dryness, dyspareunia and urinary symptoms of urgency, dysuria and recurrent urinary tract infections [3].
GSM is a source of major distress and morbidity in the breast cancer population. Between 50 to 75% of patients with breast cancer report symptoms of GSM [4]. In post-menopausal women with hormone-receptor positive breast cancer, symptoms of GSM may be further exacerbated by the use of aromatase inhibitors (AI) due to profound suppression of oestrogen in all tissues [5]. Therapeutic options for GSM are typically limited to topical lubricants or oestrogens and hormone replacement therapy in this population. For these women, the use of hormone-based therapies is often not an acceptable option unless symptoms of GSM are severe or refractory due to concerns regarding increased recurrence risk [6,7].
Fractional microablative CO2 (MonaLisa Touch™) laser is a non-surgical, non-hormone based therapy effective in early phase studies for treatment of GSM. Fractional CO2 laser aims to improve microcirculation below the level of the vaginal mucosa resulting in formation of new collagen on atrophic tissue [8,9]. Studies evaluating the use of three sessions of fractional CO2 laser (MonaLisa Touch®) in a general population of post-menopausal women resulted in a significant improvement in vulvovaginal atrophy and sexual function 12 weeks post treatment without any major adverse events [8,10] with clinical benefit sustained at 12 months after the last laser treatment [11]. Conversely, a more recent randomised study comparing CO2 laser to sham treatment showed no significant improvement in vaginal symptoms at 12 months. Over 50% of participants in this study had a history of previously treated breast cancer [12].
In women with hormone-positive breast cancer experiencing GSM whilst on adjuvant AI therapy, there is a clear need to explore non-hormone-based options with potential longer-term efficacy. This study evaluates the feasibility of fractional CO2 laser (MonaLisa Touch®) specifically in post-menopausal women with hormone-positive breast cancer and its impact on symptoms of GSM evaluated by clinical examination, patient questionnaires and tissue analysis of the vaginal epithelium.
Materials and Methods
Patients
Post-menopausal women aged 18 years with Stage I-III hormone-positive breast cancer on an aromatase inhibitor for a minimum of 6 months reporting GSM symptoms defined by one of more of the following: vaginal dryness, vaginal irritation or itch, vaginal discomfort or dyspareunia were eligible for enrolment. Exclusion criteria included concurrent use of hormone replacement therapy or topical oestrogens and evidence of lichen sclerosis or infection on clinical examination.
Study design and treatment
Fractional CO2 laser was delivered using the MonaLisa Touch® system. Patients received 3 treatment sessions scheduled 4 weeks apart using the 22mm diameter vaginal laser probe applied to the vaginal mucosa. Probe specifications were set to validated parameters: 30 watts with a dwell time of 1000μs, dot spacing of 1000μm and smart stack parameter from 1.0-3.0. All treatments were administered by one specialist gynaecologist trained in the CO2 MonaLisa Touch® technique.
Trial assessments
Clinician assessment, vaginal cytology, vaginal epithelial punch biopsy and patient questionnaires were undertaken at baseline and 12-weeks post treatment. Clinician assessment included a gynaecological examination and record of the Vaginal Health Index Score (VHIS). Patient questionnaires completed at baseline and 12-weeks post-treatment included the Urogenital Atrophy Questionnaire (UAQ) and Female Sexual Function Index (FSFI) [13,14].
End points
The primary end point was severity of GSM symptoms at 12 weeks post completion of laser therapy measured by the clinician-assessed Vaginal Health Index Score (VHIS) and patient-assessed Urogenital Atrophy Questionnaire (UAQ) score. The Vaginal Health Index Score (VHIS) [Appendix A] is a clinician assessment tool that evaluates the appearance of vaginal mucosa (elasticity, paleness, vaginal discharge, mucosal integrity, moisture) and vaginal pH. Each factor is scored on a scale of 1 to 5 and then summed to provide the VHI score. A score of less than 14 indicates vaginal atrophy. The Urogenital Atrophy Questionnaire (UAQ) is a standardised, validated self-reported 15-item questionnaire designed to identify and evaluate severity of symptoms secondary to GSM.
Secondary end points were cytological change in vaginal atrophy measured by the Vaginal Epithelial Maturation Index (VEMI), histological change in vaginal atrophy assessed by central pathology review and sexual function assessed by the Female Sexual Function Index (FSFI) score at 12 weeks post completion of laser therapy.
The Vaginal Epithelial Maturation Index (VEMI) is a ratio of the three cell types (parabasal, intermediate and superficial cell type) of the vaginal epithelium obtained by cytological sampling of the vaginal mucosa and examined using the Papanicolou stain. Vaginal atrophy is indicated by the predominance of parabasal cells due to the absence of oestrogenic stimulation on the vaginal epithelium [15,16]. Improvement in the VEMI is measured by an increase in ratio of superficial to parabasal cells on vaginal smear cytology collected at baseline and 12 weeks post completion of laser treatment. Histology was performed on 4mm vaginal mucosal punch biopsies at baseline and 12 weeks post-treatment and examined using routine (haematoxylin and eosin) and Periodic-acid Schiff (PAS) stains. Epithelial thickness was measured in micrometres taken between the epithelial rete, in well-oriented mucosa without squash artefact.
The Female Sexual Function Index (FSFI) is a standardised, validated self-reported 19-item questionnaire designed to assess domains of sexual functioning in clinical trials. The FSFI assesses sexual function in six domains including desire, arousal, lubrication, orgasm, satisfaction and pain, with a score <26.55 indicating female sexual dysfunction.
Statistical analysis
Evaluable patients were defined as those who completed three laser treatments all baseline and 12-week end-of-treatment assessments. Analyses were conducted using R 4.2 (https://cran.r-project.org/). Data was expressed using mean (SD) and median (Percentile 25-Percentile75) or (min-Max) for numeric normal and non-normal variables, respectively, and frequency (percent) for categorical variables. Comparison of baseline and end-of-treatment measurements, paired t-, Wilcoxon signed rank, sign and McNemar tests were undertaken where appropriate. The correlation among change in scores were assessed using Pearson correlation coefficients. The normality of data was decided on descriptive measures of distribution, skewness and kurtosis (within ±1.5, and ±2, respectively). P-values <0.05 were considered significant.
Trial Oversight
The trial protocol and all amendments were approved by the Cabrini Health Ethics Committee, Melbourne, Australia. The trial was conducted in accordance with the NHMRC Statement on Ethical Conduct in Research Involving Humans (© Commonwealth of Australia 2007), the NHMRC Australian Code for the Responsible Conduct of Research (© Australian Government 2007) and the principles laid down by the World Medical Assembly in the Declaration of Helsinki 2008. All patients provided written informed consent prior to enrolment.
Results
Patients
Between May 2017 and July 2020, 33 patients were enrolled. The study closed early due to slow recruitment and COVID-19 restrictions. Baseline characteristics are listed in Table 1. The median age was 52 years (range 32-76). A history of oophorectomy (n=15, 45%), prior chemotherapy (n=24, 73%) and bilateral mastectomy (n=9, 27%) were reported. Twenty-seven patients completed all 3 pre-planned laser treatments and post-treatment assessment (T1 n=30; T2 n=30; T3 n=28); post-Rx n=27) and eligible for final analysis. Reasons for study withdrawal (n=5) include cessation of AI (n=1), second malignancy (n=1), pain post biopsy (n=1) and unknown (n=2).
Table 1.Patient demographics
| n=33 (%) | |
|---|---|
| Age median (range) | 52 (32-76) |
| Stage | |
| I | 6 (18%) |
| II | 16 (48%) |
| III | 9 (27%) |
| Not reported | 2 (6%) |
| Aromatase inhibitor | |
| Anastrozole | 13 (39%) |
| Letrozole | 13 (39%) |
| Exemestane | 7 (21%) |
| Prior oophorectomy | 15 (45%) |
| Prior chemotherapy | 24 (73%) |
| Surgery type | |
| Wide local excision | 7 (21%) |
| Mastectomy | 13 (39%) |
| Bilateral mastectomy | 9 (27%) |
| Not reported | 4 (12%) |
| Treatment course completed | |
| Treatment 1 | 30 (82%) |
| Treatment 2 | 30 (82%) |
| Treatment 3 | 28 (85%) |
Primary end point
Patient-reported outcomes
Pre and post treatment evaluations: patient-reported vaginal dryness (100 vs 48%, p=<0.001), irritation/itch (56 vs 19%, p=0.008), pain (63 vs 11%, p=0.001) all significantly reduced in incidence at 12 weeks post completion of CO2 laser treatment (Table 2). No significant reduction in patient reported dyspareunia (89 vs 78%, p=0.371) was observed. At the 12-week post-treatment assessment, a significant increase of 52% (CI 33.6-69.7%, n=14/27, p=<0.001) of the study population reported improvement in the frequency of vaginal dryness; defined as experiencing vaginal dryness ‘none’ or ‘some’ of the time as opposed to ‘most’ or ‘all’ of the time on UAQ (Item 8, Figure 1). Significant reduction in the frequency of genitourinary symptoms such as dysuria, nocturia and vaginal odour was also observed (UAQ Item 1, 5 and 9) (all p=<0.05). No clinically meaningful improvement in most items relating to sexual function (UAQ Item 11-15) was detected on UAQ.
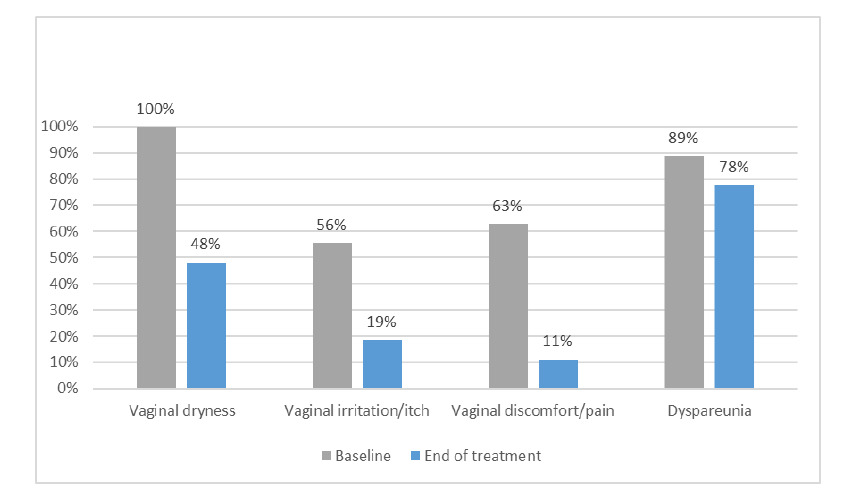
Figure 1.Baseline vs end of treatment patient reported Genitourinary Symptoms of Menopause (GSM)
Table 2.Baseline vs end of treatment Urogenital Atrophy Questionnaire (UAQ) scores
| None/some of the time (n=27) | Most/all of the time (n=27) | P-value SGT | |||
|---|---|---|---|---|---|
| Baseline | EOT | Baseline | EOT | ||
| Item 1: When I urinate, I feel a burning sensation in the opening where urine comes out | 26 | 27 | 1 | 0 | 0.008 |
| Item 2: When I have the urge or urinate, I cannot wait and must hurry to the toilet | 23 | 25 | 4 | 2 | 0.424 |
| Item 3: I leak urine when I cough, sneeze or laugh | 23 | 26 | 4 | 1 | 0.065 |
| Item 4: When I am done urinating, I do not feel like my bladder is empty | 25 | 26 | 1 | 1 | 0.754 |
| Item 5: I have to get up during the night to urinate | 13 | 16 | 14 | 11 | <0.001 |
| Item 6: I feel irritation or discomfort on the skin of my external genitals as I wipe with toilet tissue | 24 | 27 | 3 | 0 | 0.070 |
| Item 7: My vagina itches | 24 | 27 | 3 | 0 | 0.070 |
| Item 8: My vagina feels dry | 5 | 19 | 21 | 8 | <0.001 |
| Item 9: I notice an unpleasant odour from my vagina | 24 | 26 | 3 | 1 | 0.031 |
| Item 10: I have a white or creamy discharge from my vagina | 27 | 27 | 0 | 0 | 0.219 |
| Item 11: The thought of sexual activity worries me because it might cause pain in my genital area | 5 | 12 | 22 | 15 | <0.001 |
| Item 12: I am able to talk with my partners about my sexual concerns | 7 | 9 | 17 | 14 | 0.688 |
| Item 13: I am interested in sexual activity | 17 | 20 | 9 | 7 | 0.146 |
| Item 14: I desire sexual activity | 20 | 23 | 7 | 4 | 0.109 |
| Item 15: I am happy with my sex life | 22 | 24 | 4 | 3 | 0.774 |
EOT: 12-week end of treatment assessment
SGT: sign test
Clinician-assessed outcomes
The median Vaginal Health Index Score (VHIS) improved from 9.3 at baseline vs 13.4 at 12-weeks post treatment with a score 14 indicating vaginal atrophy. Median VHIS by domain at baseline vs 12-weeks post treatment was: elasticity (2/5 vs 3/5), fluid volume (2/5 vs 3/5), pH (1/5 vs 1/5), epithelial integrity (2/5 vs 4/5) and moisture (2/5 vs 3/5). The majority of evaluable patients (n=25/27) demonstrated improvement in VHIS score at 12-weeks post treatment. VHIS improved by a mean of 4.1-points at final assessment (p=<0.001), attributed to a significant improvement in epithelial integrity (p=<0.001), moisture (p=<0.001), fluid volume (p=<0.001) score and elasticity (p=0.006) scores (Appendix B).
Secondary end-points
Sexual function
FSFI score improved at 12-weeks post-treatment with median FSFI score increasing from 10.5/36 at baseline to 15.5/36 (p=0.04). An improvement in median score across most FSFI domains were observed (Table 3), however the 12-week end-of-treatment score remained <26.55, the threshold indicative of ongoing female sexual dysfunction.
Table 3.Baseline vs end of treatment FSFI domains and total score
| Sub-scales/scale | Baseline | End of treatment | P-value W | ||
|---|---|---|---|---|---|
| Median (P25-P75) | Mean(SD) | Median (P25-P75) | Mean(SD) | ||
| Desire | 4.0 (2.0-5.0) | 3.82 (1.69) | 4.0 (4.0-6.0) | 4.67 (1.57) | 0.011 |
| Arousal | 7.0 (4.0-11.0) | 6.82 (4.21) | 9.0 (4.0-13.0) | 8.59 (5.67) | 0.091 |
| Lubrication | 4.0 (3.0-9.0) | 5.33 (4.10) | 7.0 (0.0-15.0) | 7.93 (6.87) | 0.079 |
| Orgasm | 4.0 (2.0-8.0) | 5.04 (4.06) | 5.0 (0.0-10.0) | 5.93 (5.19) | 0.346 |
| Satisfaction | 4.0 (3.0-7.0) | 5.63 (3.86) | 10.0 (2.0-12.0) | 7.67 (5.20) | 0.106 |
| Pain | 3.0 (0.0-5.0) | 3.22 (3.34) | 4.0 (0.0-10.0) | 5.22 (4.77) | 0.026 |
| Total FSFI score | 10.5 (6.0-17.3) | 11.49 (6.71) | 15.5 (3.9-24.4) | 15.28 (9.28) | 0.040 |
Med: Median; P: percentile
W: Wilcoxon signed rank test
Sub-scales/scale was constructed using sum over related items
Cytology
Baseline and 12-week post treatment cytological assessment and calculation of the VEMI ratio was undertaken for each patient. No significant increase was found in the ratio of superficial to parabasal cells at 12-weeks post treatment to indicate cytological change consistent with reduction in vaginal atrophy. The median percentage of parabasal cells at baseline vs 12-week post treatment was 87% (range 1-97%) vs 84% (range 6-98%) respectively. The median percentage of superficial cells at 12-weeks post treatment remained unchanged at 0% compared to baseline.
Histology
Increase in epithelial thickness (median 0.1mm, range -0.06-0.14mm) was observed in n=15 (56%) of 12-week post treatment biopsy specimens with associated increased spongiosis and intracytoplasmic glycogen on central pathology review. The degree of chronic inflammation of the vaginal mucosa did not appear to bear any relation to the epithelial thickness. Epithelial thickness remained unchanged in n=4 (15%) and reduced in n=8 (30%) biopsies at 12-weeks post treatment (range 0.02-0.06mm). No significant correlation was found between increase in epithelial thickness at 12-weeks post treatment and clinical improvement measured by VHI score (r=0.260; 95% CI -0.069-1.0; p=0.095) and FSFI score (r= -0.016; 95% CI: -1.0-0.31; p=0.469).
Discussion
In this single-arm study, fractional CO2 laser was a feasible non-hormonal treatment for some symptoms of GSM in post-menopausal BC patients on an AI. Three courses of CO2 laser therapy resulted in a reduction in patient-reported vaginal dryness, itch and pain at 12 weeks post treatment in addition to improvements in vaginal atrophy predominantly due to an increase in epithelial integrity score on clinical assessment.
While an improvement in physiological factors were improved, our study found fractional CO2 laser was less effective in treating sexual function symptoms in women with treated breast cancer on AI therapy. Whilst total FSFI score increased post treatment, the score continued to reflect ongoing sexual dysfunction in this population. We also did not observe significant improvements in multiple sexual function domains including arousal, lubrication, orgasm or satisfaction when assessed in patient questionnaires.
In post-menopausal women with breast cancer on endocrine therapy, prior studies by Pearson, et al. [17] and Quick, et al. [18] have shown fractional CO2 laser results in both improved patient-reported symptoms of vaginal atrophy and improvement in sexual function. Marked improvement in sexual function with laser therapy were not observed in our cohort. Sexual function, desire and sexual arousability are complex and, in this study population, can be severely impacted by their diagnosis of breast cancer and its treatment such as mastectomy and endocrine therapy [19]. Population based studies report significant reduction in feelings of sexual attractiveness and comfort during sexual intimacy at 2 years post mastectomy, including those women who had undergone breast reconstruction [20].
Notably, the impact of fractional CO2 laser intervention may have been diminished in our cohort due to the prevalence of sexual dysfunction at enrolment indicated by baseline FSFI score in addition to high proportion of enrolled patients with a history of bilateral mastectomy (27%) and locally advanced disease. Our results indicate interventions that targeting vaginal atrophy and dryness alone are insufficient as sole treatment of GSM; particularly if sexual dysfunction symptoms are prominent. A holistic approach to GSM treatment may involve addressing psychological and body image factors together with sexuality in addition to interventions that target vaginal atrophy.
To date, this is the only study of women with breast cancer on AI therapy that has comprehensively evaluated the feasibility of fractional CO2 laser utilising the triad of clinician assessment, patient-reported questionnaires and tissue analysis. Fractional CO2 laser resulted in an increase in vaginal epithelial thickness in over half of evaluable patients, demonstrating objective evidence of treatment effect in women subject to the ongoing anti-oestrogenic effect of concurrent AI therapy. Interestingly, no correlation was found between the degree of epithelial thickness and clinical improvement measured by VHIS or FSFI. Results may be limited due to the small sample size and further work is needed to characterise laser-induced remodelling at the epithelial and sub-mucosal level in relation to clinical effect. Histological assessment of the submucosal tissues would require specialised staining techniques and is beyond the scope of this pilot study.
Limitations of this study are acknowledged. The small cohort, pilot design and early closure of recruitment limits any definitive conclusions being drawn. The study also was not designed to assess the duration of treatment effect or efficacy of laser treatment compared to other interventions such as lubricant or a placebo-controlled sham laser.
Whilst a 2019 meta-analysis including ten observational studies largely supports the use of fractional CO2 laser in breast cancer survivors, this intervention has not been widely adopted for the treatment of GSM due to lack of randomised and long-term safety data [21]. This issue will be addressed in the SHE CAN study (NCT04606550) that will assess fractional CO2 laser compared to topical vaginal oestrogen in women with breast cancer with GSM that will incorporate up to two years of follow up.
Conclusion
In conclusion, fractional CO2 laser is a feasible non-hormonal intervention resulting in improvement in patient-reported vaginal dryness and clinician-assessed vaginal atrophy in post-menopausal BC patients on AI therapy. The intervention was less effective for treating sexual function symptoms associated with GSM. Further work is required to explore and improve management of sexual dysfunction in this cohort.
Acknowledgements
The authors would like to acknowledge Dr William Downey and Dr Ann Niap at Cabrini Health, Malvern for assistance with histological and cytological analyses.
Conflicts Of Interest
None
References
1. Burger H. Physiological principles of endocrine replacement: estrogen. Horm Res. 2001;56 Suppl 1:82-5.
2. Castelo-Branco C, Cancelo MJ, Villero J, et al. Management of post-menopausal vaginal atrophy and atrophic vaginitis. Maturitas. 2005 Nov 15;52 Suppl 1:S46-52.
3. Portman DJ, Gass ML. Vulvovaginal Atrophy Terminology Consensus Conference Panel. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women's Sexual Health and The North American Menopause Society. Climacteric. 2014 Oct;17(5):557-63.
4. Ganz PA, Rowland JH, Desmond K. et al. Life after breast cancer: Understanding women's health-related quality of life and sexual functioning. J Clin Oncol. 1998;16:501-514.
5. Mok K, Juraskova I, Friedlander M, et al. The impact of aromatase inhibitors on sexual functioning: current knowledge and future research directions. Breast. 2008 Oct;17(5):436-40.
6. Baber R, Hickey M, Kwik M et al. Therapy for menopausal symptoms during and after treatment for breast cancer: safety considerations. Drug Saf. 2005;28(12):1085-100.
7. Cold S, Cold F, Jensen MB, et al. Systemic or Vaginal Hormone Therapy After Early Breast Cancer: A Danish Observational Cohort Study. J Natl Cancer Inst. 2022 Oct 6;114(10):1347-1354.
8. Salvatore S, Nappi RE, Zerbinati N, et al. A 12-week treatment with fractional CO2 laser for vulvovaginal atrophy: a pilot study. Climacteric. 2014 Aug;17(4):363-9.
9. Salvatore S, Maggiore L, Athanasiou S, et al. Histological study on the effects of microablative fractional CO2 laser on atrophic vaginal tissue: an ex vivo study. Menopause. 2015 Aug;22(8):845-9.
10. Perino A, Calligaro A, Forlani F, et al. Vulvo-vaginal atrophy: a new treatment modality using thermo-ablative fractional CO2 laser. Maturitas. 2015 Mar;80(3):296-301.
11. Athanasiou S, Pitsouni E, Grigoriadis T, et al. fractional CO2 laser for the genitourinary syndrome of menopause: up to 12-month results. Menopause. 2019 Mar;26(3):248-255.
12. Li FG, Maheux-Lacroix S, Deans R, et al. Effect of Fractional Carbon Dioxide Laser vs Sham Treatment on Symptom Severity in Women With Postmenopausal Vaginal Symptoms: A Randomized Clinical Trial. JAMA. 2021 Oct 12;326(14):1381-1389.
13. Lester J, Bernhard L, Ryan-Wenger N, et al. A self-report instrument that describes urogenital atrophy symptoms in breast cancer survivors. West J Nurs Res. 2012 Feb;34(1):72-96.
14. Rosen R, Brown C, Heiman J, et al. The Female Sexual Function Index (FSFI): A Multidimensional Self-Report Instrument for the Assessment of Female Sexual Function. J Sex Marital Ther. 2000; 26:191–208.
15. Nilsson K, Risberg B, Heimer G, et al. The vaginal epithelium in the postmenopause--cytology, histology and pH as methods of assessment. Maturitas. 1995 Jan;21(1):51-6.
16. Brizzolara S, Killeen J, Severino R, et al. Vaginal pH and parabasal cells in postmenopausal women. Obstet Gynecol. 1999 Nov;94(5 Pt 1):700-3.
17. Pearson A, Booker A, Tio M, et al. Vaginal CO2 laser for the treatment of vulvovaginal atrophy in women with breast cancer: LAAVA pilot study. Breast Cancer Res Treat. 2019 Nov;178(1):135-140.
18. Quick AM, Zvinovski F, Hudson C, et al. Fractional CO2 laser therapy for genitourinary syndrome of menopause for breast cancer survivors. Support Care Cancer. 2020 Aug;28(8):3669-3677.
19. Schover LR. Sexuality and body image in younger women with breast cancer. Journal of the National Cancer Institute. Monographs. 1994; (16):177-182.
20. Fallbjörk U, Rasmussen BH, Karlsson S, et al. Aspects of body image after mastectomy due to breast cancer - a two-year follow-up study. Eur J Oncol Nurs. 2013 Jun;17(3):340-5.
21. Jha S, Wyld L, Krishnaswamy PH, et al. The Impact of Vaginal Laser Treatment for Genitourinary Syndrome of Menopause in Breast Cancer Survivors: A Systematic Review and Meta-analysis. Clin Breast Cancer. 2019.
Received: December 27, 2022;
Accepted: January 16, 2023;
Published: January 18, 2023.
To cite this article : Tay R, Stewart K, Read G, et al. Treatment of Vaginal Atrophy Using Fractional Microablative CO2 Laser in Post-Menopausal Women with Breast Cancer on Aromatase Inhibitors: A Pilot Study. British Journal of Cancer Research. 2023; 6(1): 591- 596. doi: 10.31488/bjcr.180.
©2023 Tay R, et al.
